| Jun 26, 2023 |
|
|
|
(Nanowerk Information) The profitable marketing campaign of adoptive T cell therapies, a sort of immunotherapy during which immune T cells are collected from a affected person, enhanced exterior of the physique, and reinfused again into the identical affected person, particularly in opposition to blood cancers is effectively beneath manner. However bettering the power to create patient-specific T cell populations with particular traits and features might broaden clinicians’ repertoire of T cell therapies.
|
|
One strategy to method this aim is to raised perceive how T cells’ traits and features, together with their cytotoxic results on undesirable goal cells (effector T cells) or their means to recall and eradicate them in the event that they present up once more (reminiscence T cells), are formed by the mechanical resistance of the tissues they encounter whereas infiltrating them.
|
|
The mechanical options of tissues, for instance, bone, muscle, totally different inside organs, and blood, can fluctuate extensively, and pathological tissues resembling tumor lots or fibrotic tissues are mechanically considerably totally different from wholesome tissues.
|
|
Now, a analysis staff on the Wyss Institute for Biologically Impressed Engineering at Harvard College and Harvard John A. Paulson Faculty of Engineering and Utilized Sciences (SEAS), led by Wyss Core College member David Mooney, Ph.D., took a novel biomaterials method to research the impact of tissue mechanics on the state of T cells.
|
|
By engineering a three-d mannequin of the extracellular matrix (ECM), produced by cells which are accountable for tissues’ totally different stiffnesses and viscoelasticities, they had been capable of tune each parameters independently. This enabled them to exhibit a definite impression of tissue viscoelasticity on T cell improvement and performance in vitro and in vivo, and to determine a molecular pathway driving the phenomenon.
|
|
The findings are reported in Nature Biomedical Engineering (“Technology of functionally distinct T-cell populations by altering the viscoelasticity of their extracellular matrix”).
|
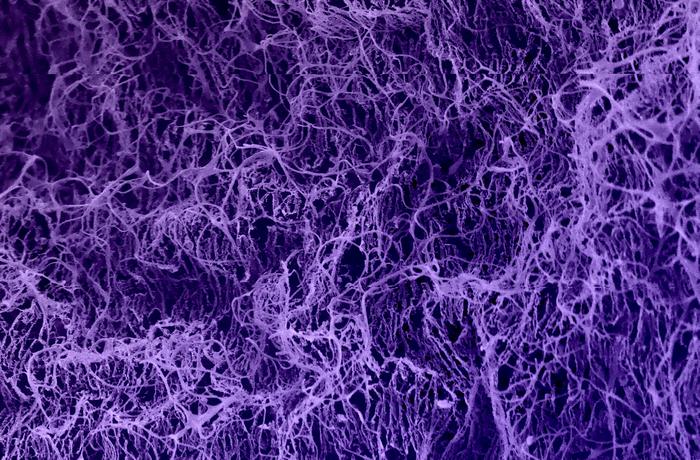 |
| This cryoEM picture reveals an ECM-mimicking hydrogel with room for T cells to go via and connect to the particularly engineered collagen matrix. (Picture: Wyss Institute at Harvard College)
|
|
Mechanical resistance comes within the type of “stiffness,” a tissue’s (or any materials’s) resistance to instantaneous deformation, and “viscoelasticity,” the kind of leisure it reveals over time following its deformation. Defined in bodily phrases, a viscous (fluid) materials, like honey, is extra prone to circulate, whereas an elastic (stable) materials returns extra quickly to its unique form, like a rubber band after stretching – and this holds true for tissues that are composed of each stable and fluid parts.
|
|
“Importantly, the phenotypes, features, and gene expression packages of T cells educated in variations of the system correlated effectively with these we present in T cells in mechanically distinct tissues from sufferers with most cancers or fibrosis,” mentioned Mooney who is also the Robert P. Pinkas Household Professor of Bioengineering at SEAS, and leads the Wyss Institute’s Immunomaterials Initiative. “Our research offers a conceptual foundation for future methods aiming to create functionally distinct T cell populations for adoptive therapies by selectively tuning mechanical enter supplied by biomaterials-based engineered cell tradition programs.”
|
Mimicking tissue mechanics in a dish
|
|
Key to their discoveries was the staff’s engineering of a tunable ECM mannequin, during which they centered on a sort of collagen that they discovered to be key to dictating the mechanical habits of various tissues. Collagen is a serious ECM protein secreted by virtually all cells within the physique. Particular person collagen protein molecules are naturally organized into crimped fibrils that mixture additional into fibers by chemically cross-linking themselves. Every fibril might be thought of a mechanical spring, and every fiber as an meeting of springs. An ECMs stiffness is determined by how densely it’s filled with collagen molecules, whereas its distinct viscoelasticity is determined by how densely collagen molecules are cross-linked to one another.
|
|
To imitate pure collagen-based ECM, the staff fabricated hydrogels whose stiffness they might tune by various the focus of collagen molecules: fewer numbers of collagen molecules produced decrease stiffness and better numbers, increased stiffness. Independently, viscoelasticity grew to become tunable by various the quantities of an artificial cross-linker molecule that additional networked the collagen molecules. Extra extremely cross-linked collagen molecules produced extra elastic hydrogels. The ensuing ECM-mimicking hydrogels equally allowed the attachment of pre-activated T cells however, importantly, enabled their stimulation with particular mechanical alerts.
|
|
“To our information, that is the primary ECM mannequin that enables researchers to review T cells with stiffness from viscoelasticity decoupled, and thus allows us and others sooner or later to research how immune and different cells could be mechanically regulated,” mentioned co-first creator Yutong Liu, Ph.D., who was a graduate pupil in Mooney’s group. “The system’s outlined and uniform mechanical stimulation is vastly totally different from how T cells are often cultured – cells that connect to the underside of a tradition dish encounter a extremely inelastic floor, whereas these remaining in suspension are surrounded by the viscous medium.”
|
Pure penalties of mechanical motion
|
|
The staff carried out an in depth evaluation of T cells uncovered to totally different viscoelastic situations. “T cells that skilled a extra elastic collagen matrix had been extra prone to become ‘effector-like T cells,’ whereas T cells that skilled a extra viscous ECM matrix fairly grew to become ‘memory-like T cells,’” mentioned co-first creator Kwasi Adu-Berchie, Ph.D., who accomplished his Ph.D. in Mooney’s lab and is at the moment a Translational Immunotherapy Scientist on the Wyss Institute. “Importantly, we discovered {that a} T cell’s state, ensuing from the viscoelasticity of a matrix, much more so from extra elastic, much less viscous hydrogels, turns into long-term imprinted, because the cell retains a reminiscence of that particular matrix after being transferred to a special one. This might have broad implications for future cell manufacturing.”
|
|
Gene expression evaluation led the staff to the exercise of a transcription issue generally known as AP-1 that hyperlinks T cells’ reception of a extra elastic, much less viscous mechanical setting to a extra effector-like gene expression program. The variety of AP-1 complexes with particular compositions was elevated, and genes relying on them for his or her expression had been enriched, not solely in T cells remoted from extra elastic hydrogels, but additionally in T cells remoted from sufferers’ most cancers and fibrotic tissues, that are stiffer and extra elastic than neighboring wholesome tissues. After they inhibited considered one of AP-1’s parts with a drug, the results of a extra elastic collagen matrix on T cells had been prevented.
|
|
To research how totally different mechanical stimulations and T cells’ predicted gene expression signatures translated into precise traits and features, the staff used therapeutic CAR-T cells engineered to bind a selected antigen of a human lymphoma cell line. CAR-T cells that had been stimulated in a extra elastic collagen matrix in vitro exhibited a stronger means to kill lymphoma cells. Additionally in vivo, CAR-T cells stimulated in a extra elastic matrix, and adoptively transferred into mice with the identical kind of lymphoma, had been considerably extra able to decreasing tumor burden within the animals and increasing their lives than CAR-T cells uncovered to a much less elastic matrix.
|
|
“This research merges three seemingly disparate fields, biomaterials, immunotherapy, and mechanobiology, to develop a wholly new type of biomaterials-based mechanotherapeutic. It’s straightforward to see how these findings can probably open up new avenues to enhance adoptive T cell therapies for sufferers sooner or later,” mentioned Wyss Founding Director Donald Ingber, M.D., Ph.D., who can also be the Judah Folkman Professor of Vascular Biology at Harvard Medical Faculty and Boston Youngsters’s Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at SEAS.
|

