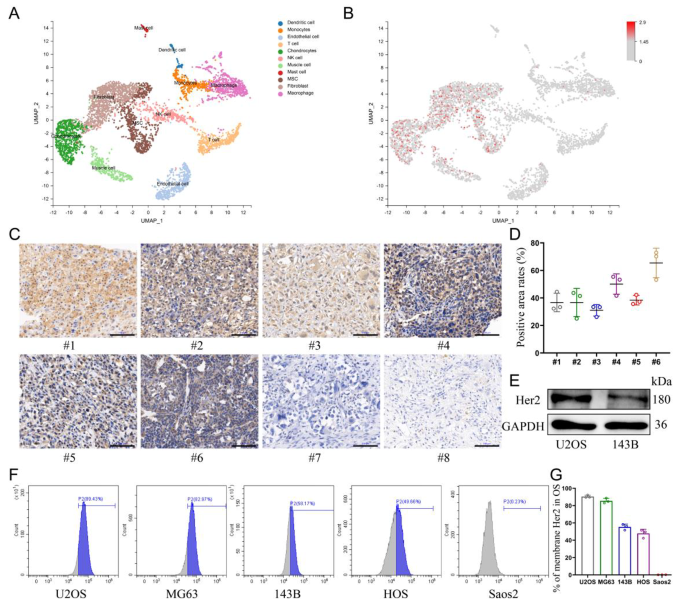
Validating the expression of Her2 in OS
The distribution of Her2 expression in osteosarcoma was explored by single-cell sequencing, which confirmed that Her2 was solely aberrantly expressed in cell populations of malignant origin (Fig. 1A, B). Due to this fact, the choice of Her2 as a goal can be anticipated to enhance the tumor-homing capability of nanomaterials and cut back the toxicity to regular tissues. Furthermore, the expression ranges of Her2 in osteosarcoma tissues from totally different sufferers have been additionally distinct (Extra file 1: Fig. S1). Immunohistochemical evaluation of samples collected from eight sufferers with OS revealed that six of them displayed Her2 expression in tumor tissues (Fig. 1C, D). 5 human-derived OS cell strains have been analyzed for Her2 expression by move cytometry, and the outcomes confirmed that 4 of them have been optimistic (Fig. 1F, G). The outcomes of western blotting confirmed the next abundance of Her2 expression in U2OS cells than in 143B cells, which was additionally according to the outcomes of move cytometry (Fig. 1E).
Validating the expression of Her2 in OS. (A) UMAP visualization of cells analyzed by scRNA-seq and built-in throughout 5 major osteosarcomas. Clusters have been annotated for his or her cell sorts as predicted utilizing canonical markers. (B) Log-normalized expression of ERBB2(Her2). (C) The expression of Her2 in osteosarcoma derived from totally different sufferers assessed by immunohistochemistry assay. Scale bar: 100 μm. (D) Share of Her2-positive areas per osteosarcoma tissue. (E) Expression of Her2 in U2OS and 143B cells decided by Western blot. (F, G) The expression of Her2 in numerous osteosarcoma cell strains was detected by move cytometry
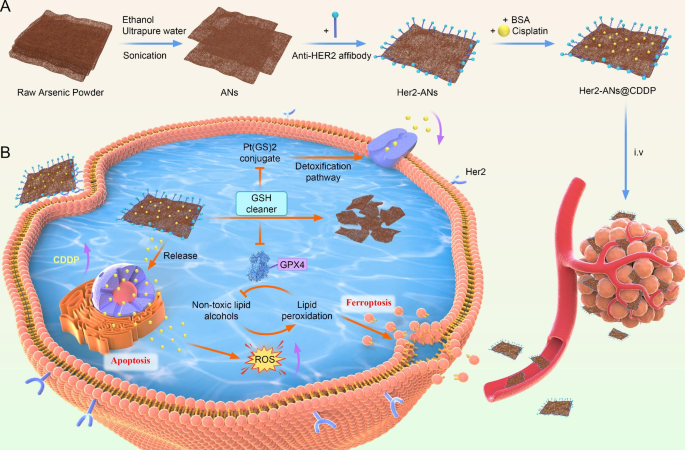
Synthesis and characterization of Her2-ANs
With the emergence of nanomedicine, there was fast growth within the software of nanomaterials to the prognosis and therapy of tumors [20]. Nonetheless, the results of medical translation is that only some nanomaterials that may be utilized to medical oncology therapies. The primary considerations are in regards to the intrinsic biosafety of supplies and the unintended effects brought on by the irregular accumulation of supplies in vivo [10]. In 1996, it was demonstrated that arsenic trioxide as a single agent may induce full remission of acute promyelocytic leukemia with solely gentle myelosuppression [16, 27]. Nonetheless, the therapeutic impact of arsenic trioxide in stable tumors is way from passable as a result of lack of focusing on [18]. On this research, we now have efficiently ready ANs by a liquid part exfoliation approach. The whole artificial route of ANs is proven in Scheme 1 A.
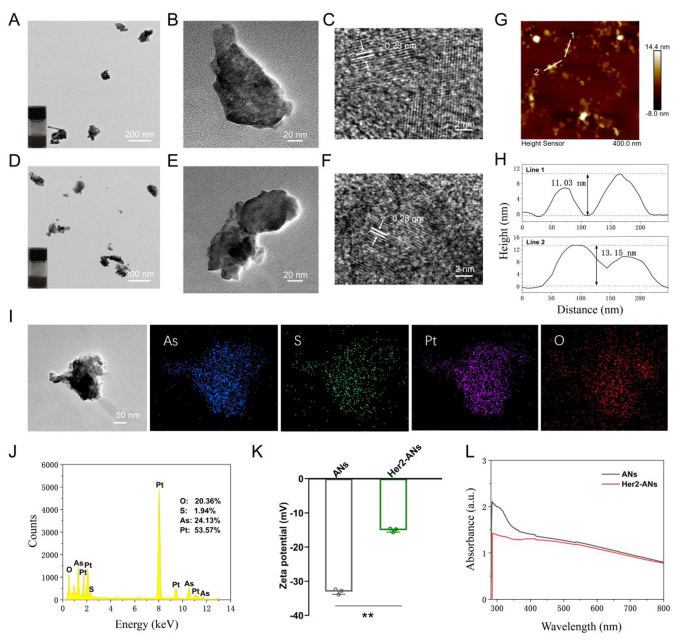
Transmission electron microscope (TEM) photographs present that the ANs exhibit ultrathin nanosheet morphology (Fig. 2B). The TEM photographs reveal that the dimensions of ANs is about 131 nm (Fig. 2B), which is considerably totally different from the uncooked arsenic powder with a measurement bigger than 2 μm (Extra file 1: Fig. S3A). The morphology and top of the uncooked arsenic powder and Her2-ANs have been analyzed by AFM, and the outcomes confirmed that the peak of the nanosheets was a few dozen nanometers (Fig. 2H), which was considerably totally different from the uncooked arsenic powder with a top higher than 2 μm (Extra file 1: Fig. S3C). As well as, the distinction in absorbance between uncooked arsenic powder, ANs and Her2-ANs was analyzed by UV-Vis absorption spectroscopy. The absorbance of the uncooked arsenic powder elevated progressively between 300 and 800 nm (Extra file 1: Fig. S3D), whereas the absorbance of ANs and Her2-ANs decreased considerably on this vary (Fig. 2L). The high-resolution transmission electron microscopy (HRTEM) photographs present the presence of 0.28 nm lattice fringes (Fig. 2C, F), that are attribute of crystalline materials. The crystal constructions of the uncooked arsenic powder and ANs have been additional analyzed by Raman spectroscopy (Extra file 1: Fig. S4B, C). The Raman spectra confirmed two attribute peaks at 209.6 and 242.2 cm-1 for the uncooked arsenic powder, which was totally different from the attribute peaks for the ANs (211.4 and 244 cm-1). Because the density of anhydrous ethanol is decrease than that of ultrapure water, anhydrous ethanol can isolate oxygen throughout the technique of exfoliation. There have been additionally no attribute peaks for arsenic oxide within the Raman spectrum. Furthermore, ethanol can decrease the temperature of the synthesis system by volatilization, which may enhance the yield of nanosheets [15]. All of the above outcomes affirm the profitable exfoliation of ultrathin ANs from the uncooked arsenic powder. Digital pictures of arsenic in ultrapure water (Fig. 2A, D; insets) present that the ANs and Her2-ANs are homogeneously dispersed in answer.
Characterization of the ANs. (A) TEM picture of ANs, and {photograph} of ANs in answer (inset). (B, C) HRTEM photographs of ANs. (D) TEM picture of Her2-ANs, and {photograph} of Her2-ANs in answer (inset). (E, F) HRTEM photographs of Her2-ANs. (G) AFM picture of Her2-ANs. (H) Corresponding top profiles alongside the white strains. (I, J) The corresponding elemental mapping (I) and EDS (J) of Her2-ANs@CDDP. (Ok) Zeta potentials of ANs and Her2-ANs. (L) UV − vis spectra of ANs and Her2-ANs. (Values are introduced as means ± sd, n = 3, *P < 0.05, **P < 0.01.)
To attain lively focusing on of the nanosheets, an Anti-Her2 Affibody was linked to the floor of ANs. Anti-Her2 Affibody is a peptide with a C-terminal cysteine residue, which could be covalently certain to arsenic [28]. The mode of motion of arsenic trioxide within the therapy of promyelocytic leukemia is predicated on the direct binding of arsenic to the cysteine residues of the zinc finger situated within the promyelocytic leukemia protein [29]. When Anti-Her2 Affibody was linked to ANs, the zeta potential modified from − 33mV to -15mV, which is according to the outcomes of two earlier affibody modification research (Fig. 2Ok) [30, 31]. Because the molecular measurement of the affibody was solely 14 kDa, the dimensions of the nanosheets elevated barely after the affibody modification (Extra file 1: Fig. S4F). FTIR spectra present attribute absorption bands of ANs and Her2-ANs at totally different frequencies. After affibody modification, the absorption bands have been modified at 1650 cm-1 and 1366 cm-1 (Extra file 1: Fig. S4A) [30]. The fundamental composition of Her2-ANs was analyzed utilizing power dispersive spectroscopy (EDS) and the outcomes present that elemental sulfur was uniformly distributed in Her2-ANs after connection of the affibody (Extra file 1: Fig. S4D). As well as, a slight shift within the attribute peaks of Her2-ANs after affibody attachment was discovered by evaluating the Raman spectra of ANs and Her2-ANs (Extra file 1: Fig. S4B). These outcomes confirmed that the Anti-Her2 Affibody was efficiently linked to the floor of ANs.
Drug loading and launch
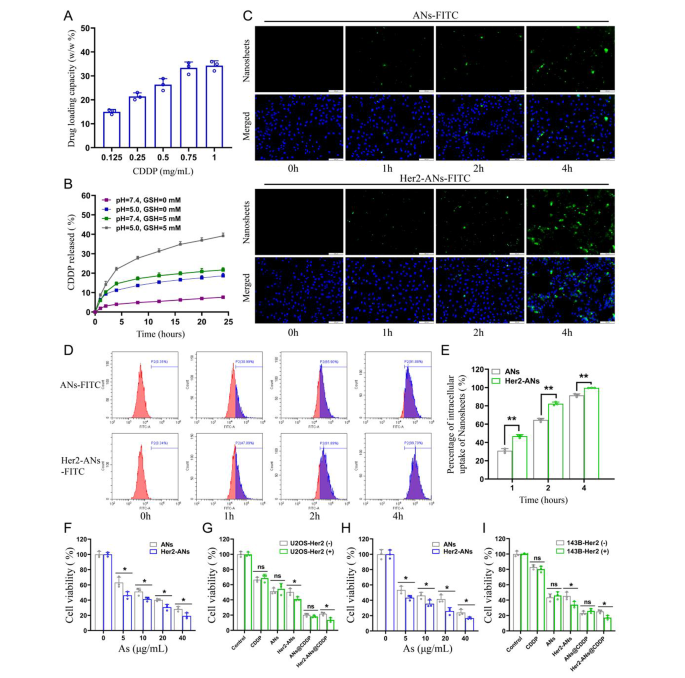
Single-element ANs as a two-dimensional nanomaterial could also be a promising service for chemotherapy medicine [15]. Outcomes from inductively coupled plasma (ICP) confirmed that the utmost cisplatin loading fee of Her2-ANs was 34.3 w/w%, which is considerably larger than the roughly 5–15% cisplatin loading fee reported in earlier nanocarrier-related research (Fig. 3A) [32, 33]. Based mostly on the outcomes of preliminary experiments, the ANs couldn’t be loaded with cisplatin with out BSA modification, which signifies that BSA assists in cisplatin loading. After 24 h of cisplatin intravenous infusion, roughly 80% of cisplatin is certain to plasma proteins, specifically to serum albumin [34]. It was demonstrated in vitro that cisplatin binding to bovine serum albumin (BSA) happens primarily by coordination with histidine or methionine aspect chains [35, 36]. As well as, the EDS mapping confirmed that S and Pt have been uniformly distributed in Her2-ANs@CDDP and the contents of As, S, O and Pt have been 24.13%, 1.94%, 20.36% and 53.57%, respectively (Fig. 2I, J).
Lots of the organic results of arsenic are mediated by the inactivation of assorted sulfhydryl-containing enzymes or proteins in cells, and sulfhydryl-containing GSH is understood to be extensively distributed in cells [13, 18]. Due to this fact, we hypothesized that arsenic would successfully deplete intracellular GSH. Firstly, we evaluated the GSH scavenging capability of Her2-ANs in vitro. After incubating of Her2-ANs with GSH for 12 h, the focus of GSH was lowered by 67% (Extra file 1: Fig. S6). Based mostly on these outcomes, we additional noticed the degradation of Her2-ANs by TEM beneath numerous GSH and pH situations. Within the absence of GSH, the morphology of Her2-ANs remained virtually unchanged (Extra file 1: Fig. S5). Nonetheless, the sides of the nanosheets turned blurred after 12 h of incubation in an surroundings with GSH (5 mM). After 24 h, the nanosheets have been noticed to degrade into smaller-sized nanofragments (Extra file 1: Fig. S5).
After figuring out the soundness of ANs beneath totally different environments, we additional explored the stimulus-responsive launch skill of Her2-ANs@CDDP beneath totally different pH and GSH situations. Within the situations with out GSH (pH = 7.4), Her2-ANs@CDDP launched solely 7.59% of cisplatin for twenty-four h, indicating that the nanocarriers have been comparatively steady earlier than reaching the tumor microenvironment (Fig. 3B). The discharge fee of cisplatin could possibly be elevated to 21.65% at 24 h by rising the GSH focus (pH = 7.4, GSH = 5 mM), which could possibly be attributed to the nanosheets decomposing within the presence of GSH. The discharge fee of cisplatin could possibly be elevated to 18.67% at 24 h by decreasing the pH (pH = 5.0, GSH = 0 mM) [32, 37]. In a simulated tumor microenvironment (pH = 5.0, GSH = 5 mM), the cumulative launch of Her2-ANs@CDDP may attain 39.18% at 24 h (Fig. 3B). Contemplating that the tumor microenvironment is characterised by excessive GSH content material and low pH, Her2-ANs@CDDP can selectively launch medicine into the inside of the tumor, which may cut back the unintended effects of cisplatin [13, 30].
In vitro tumor cell selective uptake and cytotoxicity
Since Her2 expressed on cell membranes is acknowledged particularly by Anti-Her2 Affibody, the affibody might contribute to the endocytosis of Her2-ANs [30]. We carried out mobile uptake research by laser confocal microscopy, move cytometry and ICP-MS. For U2OS-Her2(+) cells, the uptake of Her2-ANs-FITC was considerably larger than that of ANs-FITC as a result of binding of Her2 and Anti-Her2 Affibody, which was primarily manifested by the stronger inexperienced fluorescent sign exhibited within the Her2-ANs-FITC group (Fig. 3C). Evaluation by move cytometry confirmed that the uptake charges of Her2-ANs-FITC in U2OS-Her2(+) cells reached 81.89% and 99.7% at 2 and 4 h, respectively, whereas the uptake charges of ANs-FITC have been solely 65.9% and 91.88% (Fig. 3D, E). Quantitative evaluation of mobile uptake of arsenic materials by ICP-MS confirmed that Her2-positive cells took up considerably extra Her2-ANs than ANs on the similar dose, which is according to the outcomes of laser confocal microscopy and move cytometry (Extra file 1: Fig. S8A). As well as, ICP-MS outcomes confirmed that uncooked arsenic powders with sizes exceeding 2 μm have been barely taken up by the cells.
The cytotoxicity induced by ANs was assessed by CCK-8 and cell demise assays to confirm whether or not the nanosheets could possibly be utilized to tumor remedy. In contrast with the low toxicity to AML-12 regular hepatocytes, the viability of U2OS-Her2(+) and 143B-Her2(+) cells was under 20% after 20 h incubation with Her2-ANs (40 µg/mL), suggesting that arsenene nanosheets have been capable of selectively kill tumor cells (Fig. 3H and Extra file 1: Fig. S7C). As proven in Fig. 3F, H, the killing impact of Her2-ANs on Her2-positive tumor cells was considerably larger than that of ANs, which was primarily attributed to the elevated mobile uptake of nanosheets after coupling them with Her2. As well as, CCK-8 and cell demise assays confirmed that the antitumor impact of Her2-ANs@CDDP was the most effective amongst all therapy teams, suggesting a synergistic antitumor impact of ANs and cisplatin (Fig. 3G, I and Extra file 1: Fig. S7B). Collectively, these outcomes counsel that smaller sizes, sheet constructions and focused modifications are important for the uptake and cytotoxic results of arsenic supplies.
In vitro tumor cell uptake and cytotoxicity. (A) CDDP loading capacities of Her2-ANs. (B) Launch profiles of Her2-ANs@CDDP at totally different pH values and totally different GSH concentrations. (C) Intracellular uptake of ANs and Her2-ANs (labeled with FITC) within the U2OS-Her2(+) cells was measured by confocal laser scanning microscopy. Scale bar: 100 μm. (D) Intracellular uptake of ANs and Her2-ANs (labeled with FITC) within the U2OS-Her2(+) cells was measured by move cytometry. (E) Statistical analyses of the intracellular uptake fee of ANs and Her2-ANs. (F, G) Cytotoxicity of ANs and Her2-ANs in opposition to U2OS-Her2(+) cells (F). Relative viabilities of U2OS-Her2(+) and U2OS-Her2(-) cells after incubation with numerous therapies (G) (CDDP: 2 µg/mL; As ions: 10 µg/mL). (H, I) Cytotoxicity of ANs and Her2-ANs in opposition to 143B-Her2(+) cells (H). Relative viabilities of 143B-Her2(+) and 143B-Her2(-) cells after incubation with numerous therapies (I) (CDDP: 1 µg/mL; As ions: 10 µg/mL). (Values are introduced as means ± sd, n = 3, *P < 0.05, **P < 0.01.)
Mechanism underlying ANs-induced ferroptosis
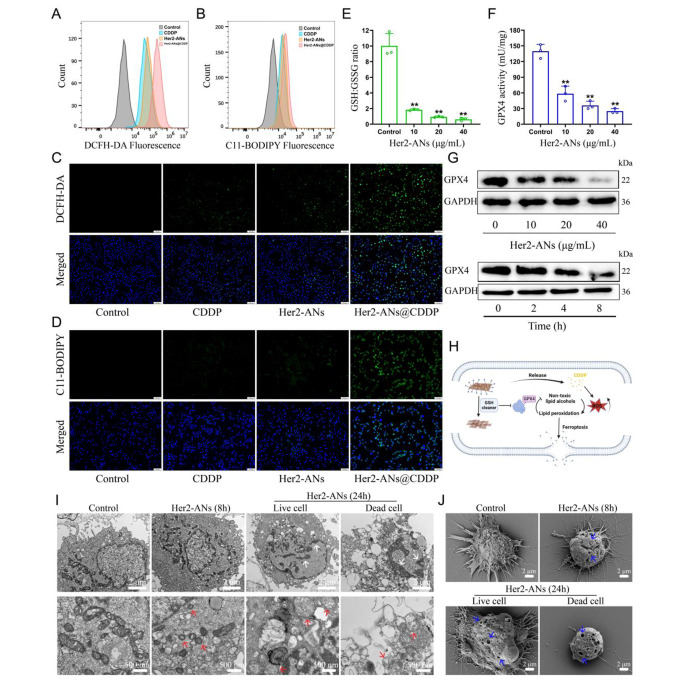
Contemplating the excellent antitumor exercise and GSH scavenging skill of ANs, we hypothesized that arsenene nanosheets may induce ferroptosis by depleting intracellular GSH and inhibiting the exercise of GPX4. Therefore, we examined the flexibility of Her2-ANs to deplete intracellular GSH. The outcomes confirmed that the degrees of intracellular GSH have been considerably decreased after Her2-ANs therapy in comparison with the management group (Fig. 4E). Since activation of GPX4 requires GSH as a cofactor, depletion of intracellular GSH will have an effect on the exercise of GPX4 [38]. The expression stage and exercise of intracellular GPX4 decreased considerably with rising focus and incubation time of Her2-ANs (Fig. 4F, G).
The GSH-centered redox system is the intracellular oxidative protection barrier that may most successfully scavenge intracellular ROS [39]. In line with the outcomes from staining utilizing the ROS probe (DCFH-DA), the extent of ROS was considerably elevated in Her2-ANs-treated cells, which was attributed to the depletion of intracellular GSH by Her2-ANs (Extra file 1: Fig. S9A, C). By activating nicotinamide adenine dinucleotide phosphate oxidase (NOX), cisplatin can produce massive quantities of intracellular O2·-, which could be transformed into H2O2 beneath the motion of superoxide dismutase. Subsequently, H2O2 catalyzes the manufacturing of ROS within the cell by the Fenton response, which is according to the outcomes of move cytometry and laser confocal microscopy photographs (Fig. 4A, C) [40, 41]. The stronger intracellular ROS fluorescence sign after Her2-ANs@CDDP therapy in comparison with cisplatin was attributed to the cisplatin-induced ROS manufacturing and lowered GSH with antioxidant exercise. Extra intracellular ROS can result in the buildup of lipid peroxides, which is a key hallmark of ferroptosis [42]. As well as, intracellular lipid peroxides can’t be transformed to non-toxic lipid alcohols as a result of inhibition of GPX4 exercise [38]. Just like the outcomes of ROS, the strongest intracellular lipid peroxide fluorescence sign was noticed after Her2-ANs@CDDP therapy (Fig. 4B, D). These outcomes counsel that the technique of depleting GSH mixed with ROS self-supplementation synergistically promote ferroptosis (Fig. 4H).
Morphologically, the buildup of extra lipid peroxides will increase membrane permeability and damages the cell membrane, finally resulting in the rupture of the cell membrane [7, 43]. The adjustments in cell membranes after therapy with Her2-ANs have been noticed by Scanning electron microscope (SEM). The outcomes confirmed pore constructions of various sizes on the floor of the cell membrane, indicating a lack of cell membrane integrity (Fig. 4J). As well as, TEM imaging of Her2-ANs-treated cells revealed a marked atrophy of mitochondria accompanied by the disappearance of cristae, a typical morphological change of ferroptosis (Fig. 4I) [7]. After 24 h, chromatin condensation was additionally noticed in cells handled with Her2-ANs, which can be attributed to DNA harm brought on by persistent and intensive intracellular oxidative stress (Fig. 4I) [44]. In contrast to beforehand reported steel nanomaterials that induce ferroptosis through the Fenton response, ANs, that are categorised as inorganic non-metallic supplies, can be utilized as a novel ferroptosis inducer by depleting intracellular GSH.
To additional validate that ANs induce ferroptosis, regulated cell demise was analyzed utilizing a number of inhibitors related to the ferroptosis pathway. As anticipated, ferroptosis inhibitor (Fer-1) and iron chelator (DFOM) prevented cell demise induced by Her2-ANs, whereas apoptosis inhibitor (Z-VAD-FMK) didn’t have any impact (Extra file 1: Fig. S8B). As well as, the addition of antioxidants (GSH or NAC) considerably inhibited the cytotoxic results of Her2-ANs. These outcomes counsel that oxidative stress and ferroptosis play a key function within the cell demise induced by Her2-ANs.
Mechanism analysis of ANs induced cell demise by ferroptosis. (A, B) Stream cytometry evaluation of the manufacturing of ROS (A) and lipid peroxide (B) in U2OS-Her2(+) cells after totally different therapies. (C, D) Confocal laser scanning microscopy imaging of the manufacturing of ROS (C) and lipid peroxide (D) ranges in U2OS-Her2(+) cells after totally different therapies. Scale bar: 100 μm. (E, F) GSH: GSSG ratio (E) and GPX4 exercise (F) in U2OS-Her2(+) cells handled with Her2-ANs. (G) Western blot outcomes for GPX4 expression in U2OS-Her2(+) cells after therapy with Her2-ANs. (H) Schematic illustration of Her2-ANs induced ferroptosis. Arsenene nanosheets may successfully deplete intracellular GSH after which induce ferroptosis by inhibiting GPX4. (I, J) TEM (I) and SEM (J) reveal nuclear, mitochondrial, and cell membrane alterations in U2OS-Her2(+) cells after therapy with Her2-ANs. (The white arrow represents DNA harm, the crimson arrow represents mitochondria, and the blue arrow represents cell membrane harm.)
ANs overcome cisplatin resistance by restoring intracellular drug accumulation
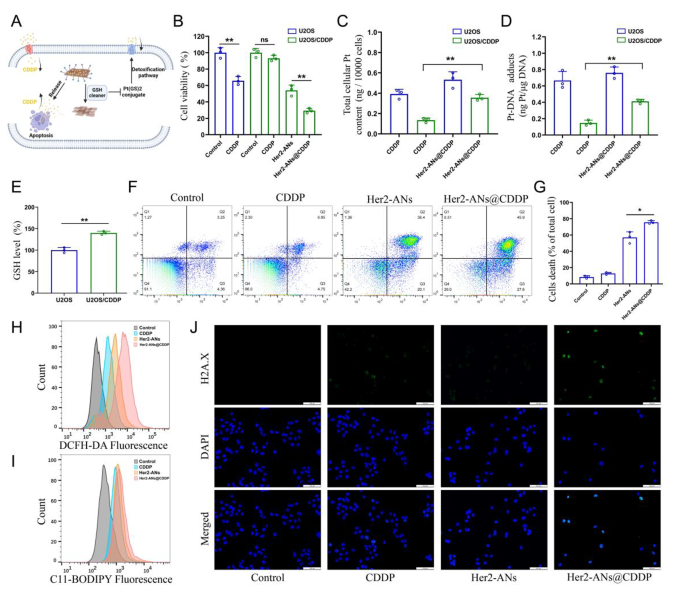
Cisplatin is the first-line chemotherapeutic agent for OS, and the event of cisplatin resistance is a problem for tumor therapy [45]. Ferroptosis is characterised by an imbalance within the intracellular redox state accompanied by elevated ranges of ROS and lipid peroxides, which is a very totally different mechanism to apoptosis [7, 46]. Inducing ferroptosis is among the greatest methods to beat apoptosis resistance [38]. To check whether or not Her2-ANs@CDDP can successfully overcome and reverse cisplatin resistance, we constructed cisplatin-resistant OS cells by stepwise drug screening. The IC50 values of cisplatin in opposition to U2OS and U2OS/CDDP cells have been 2.437 µg/mL and 12.98 µg/mL, respectively, indicating the profitable building of drug-resistant cells (Extra file 1: Fig. S11). The outcomes confirmed that Her2-ANs considerably inhibited the exercise of U2OS/CDDP cells, whereas cisplatin didn’t exhibit any cytotoxic impact (Fig. 5B). Resembling the earlier outcomes, ROS and lipid peroxidation ranges have been considerably elevated in Her2-ANs handled U2OS/CDDP cells (Fig. 5H, I). These outcomes counsel that Her2-ANs can overcome the drug resistance of U2OS/CDDP cells by inducing ferroptosis.
Cisplatin resistance is intently related to elevated ranges of intracellular GSH, and multidrug resistance-associated proteins (MRPs) are additionally concerned within the growth of cisplatin resistance [47, 48]. Cisplatin can bind GSH to type Pt(GS)2 conjugates earlier than binding to DNA, after which Pt(GS)2 is excreted from the cell mediated by MRPs, resulting in a lower in intracellular content material of the drug [5, 32]. Due to this fact, we measured GSH in U2OS/CDDP cells, and the outcomes confirmed considerably larger ranges of GSH in resistant cells in comparison with their parental cells (Fig. 5E). Based mostly on the function of GSH in cisplatin resistance and the flexibility of ANs to deplete intracellular GSH, we hypothesized that ANs might cut back Pt(GS)2 formation by reducing intracellular GSH, thereby rising intracellular accumulation of cisplatin and restoring the sensitivity of U2OS/CDDP cells to cisplatin (Fig. 5A). As proven in Fig. 5F, the best cell mortality was detected after Her2-ANs@CDDP therapy, indicating that U2OS/CDDP cells regained drug sensitivity.
To confirm whether or not the elevated cytotoxicity was on account of elevated intracellular drug accumulation, we examined the degrees of Pt within the cells and the DNA. Each whole Pt and DNA-bound Pt have been considerably elevated in Her2-ANs@CDDP-treated cells in comparison with the cisplatin-treated group (Fig. 5C, D). Cisplatin-induced DNA harm ends in the formation of γH2A.X on the web site of harm, which is an indication of a DNA double-strand break [5]. In line with the analyses of Pt-DNA, immunofluorescence confirmed the strongest γH2A.X fluorescence sign within the Her2-ANs@CDDP handled group (Fig. 5J). These outcomes point out that ANs can’t solely kill drug-resistant tumor cells by inducing ferroptosis, however can even enhance the sensitivity of drug-resistant cells to cisplatin.
ANs overcome cisplatin resistance by restoring intracellular drug accumulation. (A) Schematic illustration of arsenene nanosheets restores the sensitivity of drug-resistant cells to cisplatin. (B) Relative viabilities of U2OS and U2OS/CDDP cells after numerous therapies (CDDP: 2 µg/mL; As ions: 10 µg/mL). (C, D) Complete intracellular Pt content material (C) and DNA-binding Pt content material (D) in U2OS and U2OS/CDDP cells after incubation with CDDP or Her2-ANs@CDDP (CDDP: 2 µg/mL). (E) The extent of GSH in U2OS and U2OS/CDDP cells. (F, G) Stream cytometry evaluation of cell demise in U2OS/CDDP cells handled with numerous therapies (CDDP: 2 µg/mL; As ions: 10 µg/mL). (H, I) Stream cytometry evaluation of the manufacturing of ROS (H) and lipid peroxide (I) in U2OS/CDDP cells after totally different therapies. (J) Immunofluorescence staining of γH2A.X formation (inexperienced fluorescence) in U2OS/CDDP cells after numerous therapies (CDDP: 2 µg/mL; As ions: 10 µg/mL). Scale bar: 100 μm. (Values are introduced as means ± sd, n = 3, *P < 0.05, **P < 0.01.)
Evaluation of in vivo antitumor efficacy
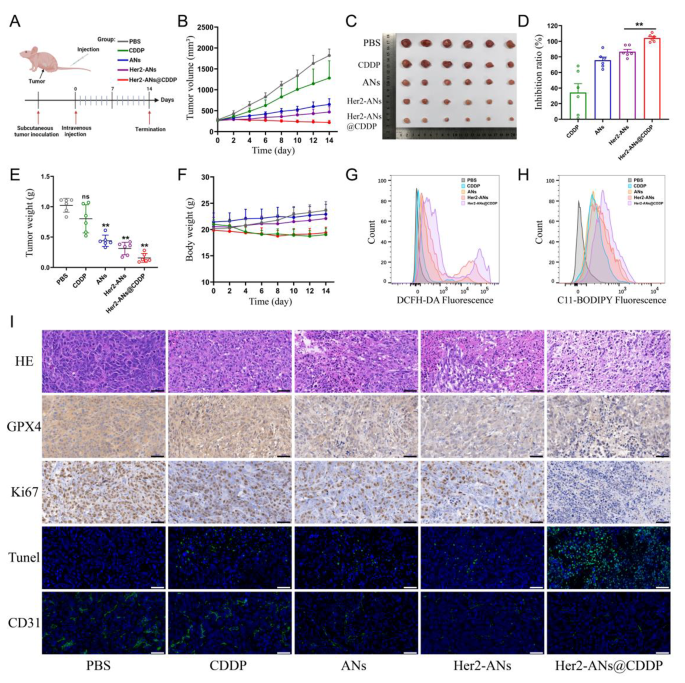
Due to the superb in vitro antitumor exercise of ANs, we additional evaluated the in vivo antitumor results of ANs. A monoclonal formation assay confirmed that Her2-ANs and Her2-ANs@CDDP may utterly inhibit the formation of tumor cell colonies in vitro (Extra file 1: Fig. S12). To analyze the tumor-homing capability of target-modified ANs, the distribution of the nanosheets in vivo was analyzed by ICP-MS. The outcomes confirmed a considerably lowered accumulation of Her2-ANs within the mononuclear phagocytic system of the liver, nevertheless, there was a big enhance within the content material of ANs inside the tumor (Extra file 1: Fig. S13). Throughout 14 days of therapy, the tumor quantity of mice within the management and cisplatin teams elevated quickly by greater than 1000 mm3, whereas the tumor volumes of mice within the different teams confirmed a big suppression (Fig. 6B). Amongst all teams, the Her2-ANs@CDDP group had the best tumor inhibition fee, which was attributed to the antitumor impact of ferroptosis induced by GSH depletion together with the focused supply of cisplatin (Fig. 6D).
To discover the mechanism by which ANs induce tumor cell demise in vivo, we measured the degrees of ROS and lipid peroxides in recent tumor tissues. As proven in Fig. 6G, H, the degrees of ROS and lipid peroxides have been considerably elevated in tumor tissues of the Her2-ANs@CDDP group. As well as, immunohistochemical staining confirmed considerably decrease expression ranges of GPX4 in tumor tissues of the Her2-ANs@CDDP group in contrast with the cisplatin group (Fig. 6I). These outcomes counsel that ANs can induce ferroptosis of tumor cells in vivo by inhibiting the expression of GPX4, leading to suppression of tumor tissue progress. As well as, pathological analyses have been carried out on sections of tumor tissues to comprehensively assess the antitumor results of Her2-ANs@CDDP. Hematoxylin and eosin (HE) staining confirmed massive necrotic areas blended with mobile particles within the tumor tissue, indicating that Her2-ANs@CDDP successfully induces tumor tissue harm (Fig. 6I). These findings have been additional confirmed by TUNEL staining, which confirmed that the tumor tissue was full of apoptotic cells. The proliferation standing of tumor cells was assessed by the expression of Ki67 protein. There was virtually full absence of Ki67 expression within the tumor tissues of the Her2-ANs@CDDP group. CD31 staining confirmed a big discount of microvessels in tumor tissue of the Her2-ANs@CDDP group, which can be attributed to important inhibition of tumor cell exercise leading to lowered angiogenesis [49]. Due to this fact, the synergistic impact of Her2-ANs@CDDP by induction of ferroptosis and apoptosis achieved a complete inhibition of OS development.
Therapeutic efficacy of Her2-ANs in vivo. (A) In vivo tumor therapy schedule. (B) Tumor progress curves of various teams. (C) Tumor pictures after numerous therapies. (D) Tumor inhibition charges of various teams. (Values are introduced as means ± sd, n = 6, *P < 0.05, **P < 0.01.) (E) The ultimate weight of tumor tissues extracted from mice after totally different therapies. (Values are introduced as means ± sd, n = 6, *P < 0.05, **P < 0.01.) (F) Physique weight adjustments in mice inside 2 weeks after totally different therapies. (G, H) Stream cytometry evaluation of the manufacturing of ROS (G) and lipid peroxide (H) within the tumor of various teams. (I) HE, immunohistochemical and immunofluorescence evaluation of tumor slices obtained from totally different teams. Scale bar: 50 μm
Biosafety evaluation of ANs
Along with assessing the in vivo antitumor results of the nanosheets, consideration was additionally targeted on the protection and reliability of the nanosheets after intravenous injection. The outcomes of a hemolysis assay confirmed that ANs didn’t trigger erythrocyte rupture at a number of focus gradients, suggesting that ANs could be administered by intravenous injection (Extra file 1: Fig. S17). In contrast with the cisplatin group, mice within the Her2-ANs@CDDP group had solely a slight lower in physique weight, which was progressively restored over time (Fig. 6F). There have been no observable necrosis, inflammatory lesions, or histological abnormalities in HE sections of the main organs of the mice (Extra file 1: Fig. S16). As well as, the hematological and blood biochemical indicators of the mice fluctuated inside the regular vary after therapy (Extra file 1: Fig. S14, Fig. S15). In conclusion, cisplatin-loaded ANs have glorious biosafety in vivo and are anticipated to be utilized to OS therapy by medical translation sooner or later.