Chemotherapy enhancement
Standard systemic chemotherapy is the spine of lung most cancers remedy. Nevertheless, the advantages of chemotherapy could be restricted as a result of points corresponding to poor aqueous solubility, non-specific mobile toxicity, low drug effectivity and antagonistic unintended effects. For instance, platinum-based compounds, corresponding to cisplatin and carboplatin, are probably the most generally used chemotherapy medication, however they usually induce undesirable off-target results, corresponding to peripheral neuropathy, nephrotoxicity, and myelosuppression [22]. Then again, functionalized nanomaterials have the potential to enhance therapeutic efficacy by minimizing non-specific mobile toxicity by way of bio-responsive response, enabling focused supply, and growing circulation time by way of PEGylation. So far, two nano-based chemotherapeutics for lung most cancers have been clinically authorized, whereas a number of formulations are at present present process medical trials (Desk 1). Abraxane (Nab-paclitaxel), an FDA-approved solvent-free nanomedicine, is a human serum albumin-bound paclitaxel (PTX) nano-formulation with a dimension of 130 nm [23]. Abraxane can dissolve into soluble albumin-PTX complexes upon injection, though some PTX might bind to different biomolecules or exist as PTX solely [24]. Notably, Nab-paclitaxel has demonstrated higher therapeutic efficacy and extra favorable drug distribution and supply than conventional solvent-based(sb)-PTX. As recognized in a section III medical trial research, Nab-paclitaxel is taken into account as a regular remedy for superior NSCLC sufferers who’ve beforehand undergone remedies [25]. Nab-paclitaxel alone (NCT04213937) or together with gemcitabine (NCT02769832) is at present being investigated in section II medical trials as a remedy routine for superior SCLC. Genexol-PM, one other FDA-approved nanoformulation of PTX, is a polymeric micelle (diameter ~ 23.91 nm) that employs monomethoxy polyethylene glycol-block-poly(D,L-lactide) (mPEG-PDLLA) to encapsulate PTX (16 wt%) [26]. Genexol-PM may also quickly dissolve into soluble albumin-bound PTX complexes after intravenously administration and have proven higher pharmacokinetics than Nab-paclitaxel as a result of PEG floor layer. Different lung most cancers particular nano-chemotherapeutics primarily based on cisplatin, docetaxel, camptothecin, and irinotecan are at present present process medical investigations (Desk 1) [27].
An growing variety of research have indicated the potential of nanomedicine in enhancing chemotherapeutic results. For instance, Solar et al. enhanced the therapeutic efficacy of PTX through the use of a multistage tumor-targeting liposome that comprises two focused peptide-modified lipids, together with cRGD-PEG2000-DSPE and KLA-PEG2000-DSPE [28]. The outcomes confirmed that the PTX liposomal nanoparticles exhibit sturdy tumor development inhibition (80.6%) and antiangiogenic results with out inducing systemic toxicity in tumor-xenografted BALB/c mice [28]. The liposomal formulation, which comprises cyclic derivatives of RGD (Arg-Gly-Asp) oligopeptides, can selectively bind to the αvβ3 integrin that’s extremely expressed in tumor cells, corresponding to lung most cancers, breast most cancers, and activated vascular endothelial cells. The formulation additionally consists of one other peptide, D-(KLAKLAK)2 (KLA), which is a positively-charged, mitochondria-targeting sequence that may goal and disrupt the mitochondrial membrane.
Along with artificial lipids or peptides, pure bio-membrane (together with mobile membranes and extracellular vesicles) coatings have proven nice success in bettering drug supply in varied tumor sorts together with lung most cancers [29]. For instance, through the use of purple blood cell membrane (RBCm) wrapping expertise, Gao et al. constructed RBCm wrapped pH-sensitive poly(l-γ-glutamylcarbocistein)-PTX nanoparticles to delay the blood circulation time and permit for well timed launch of PTX in acidic tumor microenvironment (TME) [30], and these RBCm wrapped nanoparticles exhibited considerably stronger antitumor impact (P < 0.001) in NSCLC tumor-bearing mice than non-wrapped nanoparticles. Furthermore, Agrawal et al. ready milk-derived exosomes and loaded them with PTX (Exo-PTX) [31]. Oral administration of Exo-PTX in nude mice bearing human lung carcinoma xenografts resulted in important inhibition of tumor development (60%) and remarkably decrease systemic and immunologic toxicities as in comparison with intravenously injected PTX [31].
One other promising method entails using nanocarriers to mix totally different chemotherapeutic brokers, thus optimizing the therapeutic efficacy whereas minimizing additive unintended effects. As an example, Jiang et al. developed combretastatin A4 nanodrug (CA4-NPs) and matrix metalloproteinase 9 (MMP9)-activated doxorubicin prodrug (MMP9-DOX-NPs) (Fig. 1A) [32]. The sequential supply of CA4-NPs and MMP9-DOX-NPs elevated tumor-selective drug launch by amplifying MMP9 expression and enhanced antitumor efficacy with a tumor inhibition price of 88.2% (Fig. 1B,C). The cooperative technique resulted in a 1.8-fold enhance in efficacy in contrast with the noncooperative controls (Fig. 1D) [32]. Furthermore, Wang et al. first synthesized a glutathione-responsive and pH-responsive cisplatin prodrug (PEG-ADH-DPA-DDP) after which constructed cisplatin prodrug and PTX co-loaded nanoparticles (DDP-P/PTX NPs) [33]. The nanoparticles exhibited redox-sensitive and pH-triggered drug launch in a murine mannequin of lung most cancers, leading to excessive tumor distribution, low systemic toxicity, and synergistic anti-tumor results [33]. Equally, Liu et al. engineered lipid-polymer hybrid nanoparticles (LPNs) as a co-delivery system of PTX and triptolide. The mixed benefits of each polymeric nanoparticles and liposomes resulted in synergetic antitumor results with minimal systemic toxicity [34].
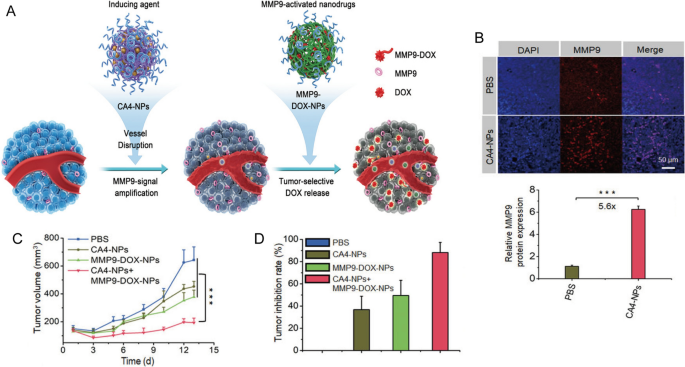
Reprinted with permission [32]. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
A Scheme illustration of cooperative most cancers remedy by combining CA4 nanodrug and MMP9-activated DOX prodrug nanomedicine. B Immunofluorescence photos and quantitative evaluation of MMP9 in tumor tissues. C Tumor quantity and D tumor inhibition price of 4T1 tumor mannequin.
Radiotherapy sensitizer
Radiotherapy is without doubt one of the mostly used therapies for lung most cancers sufferers, no matter their illness stage [35]. The forms of radiation remedy used embody systemic, exterior beam and inside radiotherapies. Nevertheless, the general survival price continues to be removed from supreme for sufferers handled radiotherapy. That is largely as a result of growth of radioresistance, which is usually attributable to the prevalence of hypoxia and the plasticity of most cancers stem cells [36, 37]. Thus, it’s of nice significance to design radiosensitizers particular for lung most cancers radiotherapy purposes. Nanomaterials containing components with excessive atomic quantity (Z) have emerged as supreme radiosensitizers. These nanoplatforms can enhance the dose of radiation vitality absorbed within the tumors and thus enhance the therapeutic efficacy of standard radiotherapy [38]. Nanoparticles primarily based on Au (gold), Bi (bismuth), and Lu (lutetium-177) have been acknowledged as potential radiosensitizing supplies as a result of their excessive X-ray absorption and distinct physicochemical properties [39, 40]. For instance, Zhuang et al. developed small interfering RNA (siRNA)-Specificity Protein 1 (SP1) loaded AuNPs (AuNPs-si-SP1) [41]. SP1 is a transcription issue overexpressed in lung most cancers sufferers and was predicted to have a binding web site with granzyme B. Outcomes confirmed that the nanoparticles elevated the radiosensitivity of lung most cancers by lowering cell viability and survival by inhibiting SP1 and upregulating granzyme B [41]. Furthermore, Xiao et al. loaded adipose-derived mesenchymal stromal cells with radiosensitive bismuth selenide (Bi2Se3) NPs (AD-MSCs/Bi2Se3) to allow focused radiotherapy of NSCLC [42]. AD-MSCs/Bi2Se3 may effectively accumulate at tumor web site and additional improve radiotherapeutic efficacy upon X-ray irradiation in orthotopic A549 tumor-bearing mice [42]. As well as, radiotherapy supported by selenium nanoparticles (nano-Se) may induce cell apoptosis and forestall NSCLC proliferation, migration, and invasion [43].
In medical observe, radiotherapy is often mixed with different therapies, corresponding to chemotherapy and focused remedy, to realize higher outcomes for lung most cancers sufferers. Chemoradiotherapy with cisplatin and etoposide is without doubt one of the major remedy strategies for each SCLC and NSCLC sufferers. Nevertheless, the excessive interstitial stress, low blood circulate, hypoxia, and acidosis within the TME can restrict drug accumulation within the tumor tissues [44, 45], additional reducing the efficacy of radiotherapy. Nanomaterials could also be synthesized with relative ease to supply versatility and multifunctionality that may improve radiotherapy efficacy by assuaging tumor hypoxia or bettering cytotoxic results by way of reactive oxygen species (ROS) technology [40, 46]. For instance, Zhang et al. ready biocompatible polylactic-co-glycolic acid (PLGA)-PEG polymeric NPs to co-deliver cisplatin and etoposide in a murine mannequin of NSCLC [47]. Along with radiotherapy, the therapeutic efficacy was considerably elevated in two murine lung most cancers fashions with out inducing extra toxicity [47]. In one other research, Wang et al. designed and constructed epidermal development issue (EGF)-modified doxorubicin nanoparticles (EGF@DOX-NPs) utilizing biocompatible polyethylenimine (PEI) coated polylactic acid (PLA)-PEG-PLA copolymer (Fig. 2A) [48]. They administered a single dose of 5 Gy X-ray radiation to regionally burst the tumor vasculature and promote macrophage infiltration. This technique considerably elevated the buildup of EGF@DOX-NPs within the tumor tissues (0.68 ± 0.08 µg/mL of RT + EGF@DOX-NPs vs. 0.11 ± 0.07 µg/mL of free DOX) which resulted in superior tumor inhibition results (Fig. 2B,C) [48]. As well as, in vivo micro PET/CT confirmed that the mix of radiotherapy with EGF@DOX-NPs considerably inhibited glucose metabolism of tumors (Fig. 2D). Observe that the above method might produce various tumor permeability and nano-radiosensitization results on a case-by-case foundation as a result of heterogeneity and complexity of the tumor extracellular matrix.
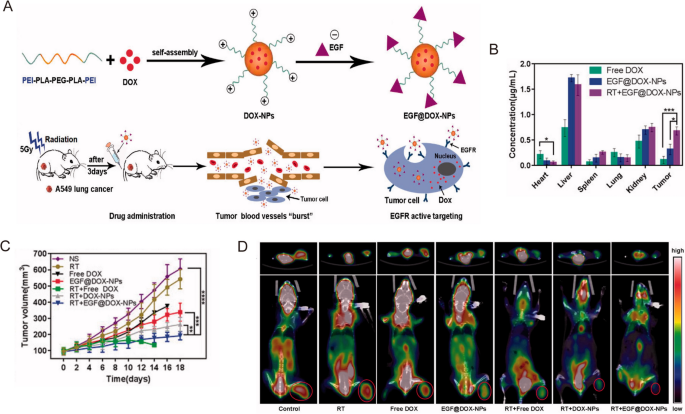
Reprinted with permission [48]. Copyright 2022, The Creator(s)
A Schematic illustration of the NPs preparation and radiation-induced drug aggregation. B DOX focus within the important organs. C Tumor quantity change of various teams in lung tumor xenografts mouse mannequin. D Consultant micro PET/CT photos of resected tumor and mice with totally different medication.
Mixture with immunotherapy
Immune checkpoint immunotherapy
Immunotherapy, particularly immune checkpoint remedy, has been extensively studied and utilized as remedy for lung most cancers prior to now few years. Immune checkpoints, which operate as unfavourable regulators of immune activation, are proteins on the floor of T cells and different immune cells [49]. Essentially the most extensively accepted ICIs embody monoclonal antibodies concentrating on CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab), or PD-L1 (durvalumab, atezolizumab, avelumab). There’s ample medical proof to assist the advantages and use of those ICIs in sufferers with lung most cancers [50, 51]. ICIs may also induce a spectrum of immune-related antagonistic results (irAEs) because of their immunologic mechanisms of motion [50]. Nevertheless, some sufferers might not reply to ICIs remedy (for instance, about 20% of NSCLC sufferers) [52]. Thus, mixture immunotherapy is a promising technique to successfully enhance the sensitivity and availability of ICIs.
It has been discovered that nanomedicines can considerably enhance therapeutic efficacy of ICIs for lung most cancers [53]. As an example, Yang et al. efficiently synthesized nanomicellar encapsulated-PTX (nano-PTX) that induced immunogenic cell loss of life (ICD) and triggered an immune response in LL/2 lung most cancers mannequin [54]. Furthermore, nano-PTX upregulated the expression of PD-L1 in immune cells and tumor cells. Thus, the co-treatment with PD-1 antibody may successfully suppress tumor development and delay survival [54]. Equally, Wang et al. constructed cisplatin-NPs by loading cisplatin inside a PLGA-graft-methoxy PEG complicated for radiation-induced ICD remedy. As-prepared cisplatin-NPs may amplify radiation and enhance ICD efficacy by way of enhanced CD8+ T cells priming and chemokine (C-X-C motif) ligand 10 (CXCL10) secretion [55]. Consequently, the mix of cisplatin-NPs, radiotherapy and anti-PD-1 considerably inhibited tumor development in comparison with the mix with molecular cisplatin drug in murine fashions of lung most cancers (major tumor quantity on day 14: 173 vs. 653 mm3) [55].
Along with T cells, PD-1 expression has been recognized in lots of different immune cell sorts, particularly tumor-associated macrophages (TAMs) [49]. For instance, Xu et al. fabricated nanodiamond-polyglycerol-doxorubicin conjugates (nano-DOX) and located that nano-DOX may induce PD-L1 in NSCLC cells and PD-1 in TAMs by the activation of the HMGB1/RAGE/NF-κB pathway [56]. They confirmed that nano-DOX and PD-1 blockade may repolarize TAMs into an M1-like phenotype, subsequently triggering synergistic antitumor impact in NSCLC xenografts [56]. In one other research, Liu et al. designed gold nanoprisms (GNPs) for each PTT and PD-L1 siRNA supply. The GNPs-hPD-L1 siRNA sevred as an efficient nanoplatform for downregulating PD-L1 expression and photoacoustic imaging in addition to photothermal brokers within the meantime. Thus, the synergistic therapeutic results of phototherapy and immunotherapy might be realized in each HCC827 cell line and xenograft mannequin [57]. Zhou et al. discovered that integrin β3 (β3-int) is very upregulated in NSCLC sufferers with spinal metastasis (NSCLC-SM), and the inhibition of β3-int would result in the ubiquitin degradation of PD-L1. Thus, β3-int serves as a possible goal for blockade immunotherapy [58]. Within the research, they functionalized mesoporous silicon nanoparticles with Arg-Gly-Asp-d-Tyr-Lys (RGDyK), a β3-int inhibitor, and zinc protoporphyrin (ZnPP) (Z@M-R) (Fig. 3A). Z@M-R confirmed environment friendly promotion impact on ubiquitination degradation of PD-L1 in A549 cells (Fig. 3B). By way of ZnPP-induced PDT and RGDyK-induced PD-L1 blockade, Z@M-R nanoparticles elevated CD8+ cytotoxic T-cell proliferation and exhibited important immunotherapeutic results owing to the elevated infiltration of CD4+ and CD8+ T cells (greater than 30 instances in comparison with saline controls) within the tumor tissues of NSCLC-SM fashions (Fig. 3C,D) [58].
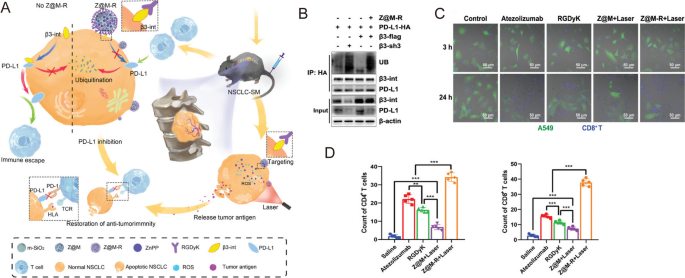
Reprinted with permission [58]. Copyright 2022, Wiley-VCH GmbH.
A Schematic illustration of the organic mechanism of Z@M-R. B Z@M-R selling PD-L1 ubiquitination in A549 cells. C Immunofluorescence photos of β3-int-overexpressing A549 cells and activated CD8+ T cells co-culture methods. D Quantitative evaluation of CD4+ and CD8+ T cells in tumor tissues with totally different remedies.
Most cancers vaccines
Moreover ICIs, quite a lot of immunotherapies involving varied cytokines, agonists, antagonists, antibody-drug conjugates, nucleic acids and so forth have been investigated as potential remedies for lung most cancers in each preclinical and medical research [51]. Nevertheless, the uncontrolled biodistribution of those small molecules usually induces dose-dependent irAEs, corresponding to cytokine-releasing storms, thus hampering their medical use. Then again, most cancers vaccines have been designed to delay the antitumor response with out inducing antagonistic off-target results [59]. Specifically, rising nanoplatforms have proven to enhance the efficacy of most cancers immunotherapy by way of particular TME regulation, sustained antigen launch, and enhanced immune stimulation [53, 60]. For instance, Hsieh et al. developed zero-valent-iron nanoparticles (ZVI-NPs) that present immunity towards lung most cancers by reworking TAMs to antitumor M1 phenotype each in vitro and in vivo (Fig. 4A, B). In comparison with management, ZVI-NPs may inhibite tumor development by 4 instances (Fig. 4C)and improve lymphocytic immunity by reducing the proportions of PD-1+ cells and CTLA4+ cells in tumor-infiltrating CD8+ T cells by 20% and lowering the quantity of regulatory T cells (Tregs) by half (Fig. 4D) [61].
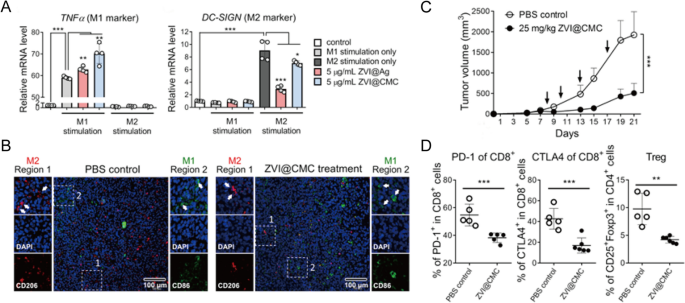
Reprinted with permission [61]. Copyright 2021, The Creator(s)
A Expressions of TNFα (M1 marker) and DC-SIGN (M2 marker) measured by RT-qPCR in THP-1 macrophages handled with ZVI-NPs. B Immunofluorescent staining of macrophages in tumor tissue sections. C Tumor quantity adjustments of various teams. D The proportions of various T cells in tumors analyzed by circulate cytometry evaluation.
In one other research, Koh et al. designed a novel most cancers vaccine primarily based on nanoemulsion (NE) and TLR7/8 agonist (R848) that would convert myeloid-derived suppressor cells (MDSCs) into mature myeloid cells in addition to M2 macrophages into M1 macrophages [62]. Notably, this most cancers vaccine confirmed adjuvant therapeutic efficacy towards anti-PD-1 immunotherapy. The mix remedy group exhibited stronger antitumor capability (P = 0.0002) in contrast with anti-PD-1 monotherapy. The management and anti-PD-1 teams confirmed tumor development, whereas the mix group confirmed tumor-free survival in 4 out of 9 mice [62]. Equally, Ye et al. synthesized a TME-modeling neobavaisoflavone nanoemulsion [63]. Such nanoemulsion may induce phenotypic change in macrophages (M2 to M1), enhance pure killer (NK) cell quantity, and scale back the infiltration of immune-suppressive cells (e.g. Tregs and MDSCs). Consequently, lung most cancers development was suppressed by practically 3 instances in comparison with the one neobavaisoflavone remedy group in an A549 lung most cancers xenograft mannequin [63].
Nanomedicine formulations can promote most cancers vaccine-like results by way of a number of stimulations and long-term launch. For instance, superior injectable good hydrogels (ISHs) have been employed as a most cancers vaccine platform. After administration, managed degradation of ISHs may guarantee persistent launch of antigen-loaded nano-sized polyplexes and granulocyte-macrophage colony-stimulating issue (GM-CSF), thereby successfully suppressing human lung carcinoma in vivo [64]. Equally, Oh et al. utilized gelatin-based hydrogel to co-deliver DCs, oncolytic adenovirus co-expressing interleukin (IL)-12, and GM-CSF, which resulted in sustained, synergistic most cancers vaccine therapeutic results [65].
Ablative remedy optimization
Radiofrequency ablation (RFA)
RFA is a one-step, minimally invasive process that’s nicely tolerated in medically inoperable sufferers. RFA guided by computed tomography (CT) is possible for treating early lung most cancers in addition to pulmonary metastases from a variety of major tumors with restricted lung tissue damages [66, 67]. Nonetheless, the efficacy of RFA is restricted as a result of threat of incomplete ablation, so extra intervention is commonly required. Native RFA remedy mixed with systemic remedy, corresponding to chemotherapy, focused remedy, and immunotherapy, has been demonstrated to extend the survival price of lung most cancers sufferers by lowering lung most cancers recurrence [68]. Furthermore, it has been acknowledged that RFA can probably set off particular immune response by way of tumor antigen launch. As an example, Xu et al. urged that intratumoral administration of CpG (TLR9 agonists) adopted by RFA may improve RFA-induced cytotoxic T lymphocyte (CTL) responses. This mixed remedy halted the expansion of major RFA-treated and distant untreated tumor in addition to the unfold of lung metastasis [69]. Apparently, Li et al. confirmed that native RFA mixed with melatonin (MLT) may considerably enhance the medical outcomes of early lung most cancers sufferers with a number of pulmonary nodules [70]. Native RFA remedy first initiated the recruitment of NK cells to the tumor web site, and MLT additional promoted the antitumor immune response from RFA-induced NK cells recruitment, thus exerting synergistic inhibitory results on lung tumor development [70]. Primarily based on these findings, the mix of nanomedicine and RFA might thus give option to improved antitumor immune response. For instance, Yang et al. co-encapsulated lipoxidase and hemin (an iron catalyst) in PLGA utilizing a CaCO3-assisted double emulsion technique (HLCaP) [71]. With RFA, the HLCaP nanoreactors induced efficient lipid peroxidation that resulted in immunogenic cell loss of life. This remedy additional mixed with anti-PD-1 immunotherapy resulted in development inhibition of residual tumors, thus stopping tumor recurrence and lung metastasis [71].
Phototherapy
Phototherapy is a non-invasive process that serves instead remedy for sufferers with localized central NSCLC who’re unable to endure surgical resection [16]. Phototherapy, together with photothermal and photodynamic remedy (PTT and PDT), makes use of laser irradiation to comprehend therapeutic results. Upon near-infrared (NIR) laser irradiation, PTT achieves localized heating to ablate tumor tissues [72], whereas PDT generates free radicals and ROS to induce mobile damages [73]. Nevertheless, it’s difficult to realize fascinating phototherapeutic results with standard small molecular brokers as a result of their non-specificity, low photoconversion effectivity, and poor bioavailability. Then again, nanomaterials could be modified to hold photothermal brokers (PTA) or photosensitizers, and their designs could be tuned to encourage tumor accumulation. So far, a broad array of nanoprobes have been studied as PTT and PDT brokers, together with magnetic nanoparticles, metallic nanoparticles, carbon nanotubes, two-dimensional (2D) supplies, metal-organic frameworks (MOFs), quantum dots, NIR dyes encapsulated nanoparticles, and semiconducting polymer nanoparticles [74,75,76]. As an example, black phosphorus nanosheets (BP NSs), a 2D nanomaterial with excessive floor space and unfavourable cost, are extremely biocompatible with the power to generate warmth and ROS upon laser irradiation, demonstrating the feasibility of utilizing this agent for each PTT and PDT [77].
Nonetheless, it’s nicely accepted that the whole elimination of tumor tissues shouldn’t be doable with phototherapy alone, and this will trigger tumor recurrence as a result of presence of residual tumor cells. Thus, appreciable analysis efforts have centered on combining phototherapy with different forms of remedy, corresponding to chemotherapy and immunotherapy, whereas incorporating nanomedicine to additional mediate and optimize the therapeutic efficiency [78]. For instance, Zhang et al. developed a novel nanomedicine formulation by loading BiOI@CuS nanoparticles with doxorubicin, aspirin phenacetin, and caffeine (APC) [79]. When subjected to 980 nm laser irradiation, this nanosystem triggered the discharge of DOX and APC by way of photothermal heating, thereby bettering the therapeutic final result whereas minimizing antagonistic unintended effects [79]. To assemble a flexible nanoplatform, Lai et al. used peptide nanotubes to biomineralize Cu2 − xS nanoparticles and built-in them with an oxaliplatin prodrug (Pt-CuS-PNTs) by way of covalent interactions (Fig. 5A) [80]. Upon 808 nm laser illumination, this nanoplatform resulted in important tumor hyperthermia and ROS technology, which contributed to the inhibition of tumor development and lung metastasis in a B16-F10 melanoma mannequin (Fig. 5B, C) [80].
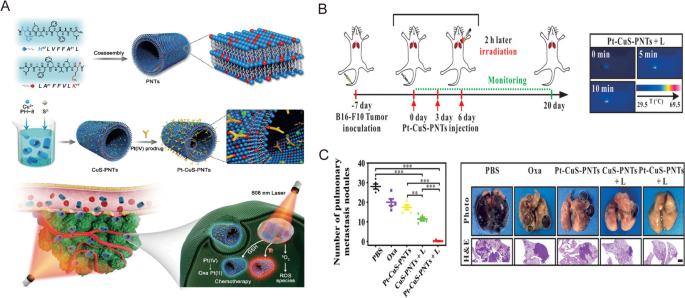
Reprinted with permission [80]. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
A Schematic illustration of Pt–CuS–PNT preparation and the mix most cancers remedy. B Experimental scheme and infrared thermal photos of the mix remedy of lung metastatic melanoma mice mannequin. C Quantitative evaluation of the lung metastatic nodules and consultant photos and H&E of the lung tissues.
The immune activating functionality of phototherapy has prompted using varied nanomaterials as most cancers vaccines by way of photo-immunotherapy. For instance, Liu et al. developed a novel most cancers therapeutic vaccine by encapsulating black phosphorus quantum dots inside exosomes and utilized the nano-vaccine in a murine subcutaneous lung most cancers mannequin [81]. This vaccine demonstrated glorious PTT efficacy by activated host immunity that subsequently elevated the variety of tumor-infiltrating T-cells [81]. In one other research, Huang et al. designed an in situ photothermal nano-vaccine by co-encapsulating immune adjuvant CpG-loaded BP-Au nanosheets with an indoleamine 2,3-dioxygenase inhibitor (NLG919) [82]. The resultant tumor vaccine activated the effector and reminiscence T cells, subsequently suppressing Tregs. This immune response led to exceptional therapeutic results on each major and lung metastatic tumors [82].

