Supplies
Anhydrous copper chloride (CuCl2), 3,3’,5,5’-tetramethylbenzidine (TMB), bovine serum albumin (BSA), and sodium sulfide nonahydrate (Na2S·9H2O) have been from Macklin Biochemical (Shanghai, China). Doxorubicin (Dox), N-(3-Dimethylaminopropyl)-N’-ethylcabodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS) have been from Sigma-Aldrich (USA). Antibodies in opposition to p16ink4a (polyclonal, ab108349), B2M (monoclonal, ab75853), excessive mobility group protein 1 (HMGB-1) (ab228624), matrix metalloprotein 13 (MMP-13) (ab39012), and kind II collagen (Col-2) (ab34712) have been from Abcam (UK). The mobile senescence β-galactosidase staining package and cell counting package (CCK-8) have been from Beyotime Biotechnology (Shanghai, China). FITC-xtra and MitoROS™ 580 have been from ATT Bioquest (USA). The Evo M-MLV Reverse Transcription Reagent and SYBR Inexperienced Professional Taq HS qPCR Equipment have been from Correct Biology (China). The chondrogenic differentiation package was from Cyagen Biosciences (USA).
Synthesis of CuS NPs and B2M-CuS NPs
CuS NPs have been synthesized by a beforehand reported methodology, with some modifications [29]. Briefly, 100 µL of CuCl2 (1 M, 13.445 mg) answer was added to 10 mL of BSA (10 g L− 1) answer and stirred at room temperature (RT, 25 °C) for five min. Then, 100 µL of Na2S answer (1 M, 7.804 mg) was added dropwise over 5 min into the above combination beneath steady stirring. The combination was stirred at 90 °C till a darkish inexperienced shade fashioned, and free BSA and ions have been eliminated by centrifugation and dialysis in opposition to ddH2O.
To organize the B2M-CuS NPs, B2M antibody was conjugated to CuS NPs by an EDC/NHS response [30, 31] with a 1:3 antibody:NP molar ratio. Unmodified B2M antibody was eliminated by repeated centrifugation for 10 min at 10,000 rpm. The resultant B2M-CuS NPs have been saved at -20 °C earlier than use.
Characterization
The morphology of CuS NPs was noticed by high-resolution transmission electron microscopy (HRTEM; JEOL JEM 2100 F, Japan). The crystalline construction of CuS NPs was decided by X-ray diffraction (XRD; Rigaku Smartlab 9KW, Japan), and absorption spectra have been obtained by a spectrophotometer (Thermo Scientific Multiskan GO, USA). The valence states of Cu in NPs have been studied by X-ray photoelectron spectroscopy (XPS; Thermo Scientific Ok-Alpha). The zeta potential and hydrated particle measurement of CuS NPs and B2M-CuS NPs have been decided utilizing a nano-analyzer (Malvern Zetasizer Nano ZS90, UK).
Peroxidase-like exercise
The peroxidase-like exercise of CuS NPs was evaluated as beforehand reported utilizing TMB as a substrate [24]. For the detection of H2O2-dependent oxidation of TMB, totally different reagent mixtures have been set, together with (1) TMB + H2O2, (2) TMB + NPs, and (3) TMB + H2O2 + NPs. CuS NP options (100 µL, 100 µg mL− 1) have been diluted with HAc/NaAc buffer (0.2 M:0.2 M, pH = 4.6). Then, 100 µL of TMB answer and 100 µL of H2O2 (10 mM) in dimethyl sulfoxide (2 mM) have been added to the NP options. After incubation at RT for 30 min, the absorbance of the samples was recorded (BioTek Synergy H1, USA).
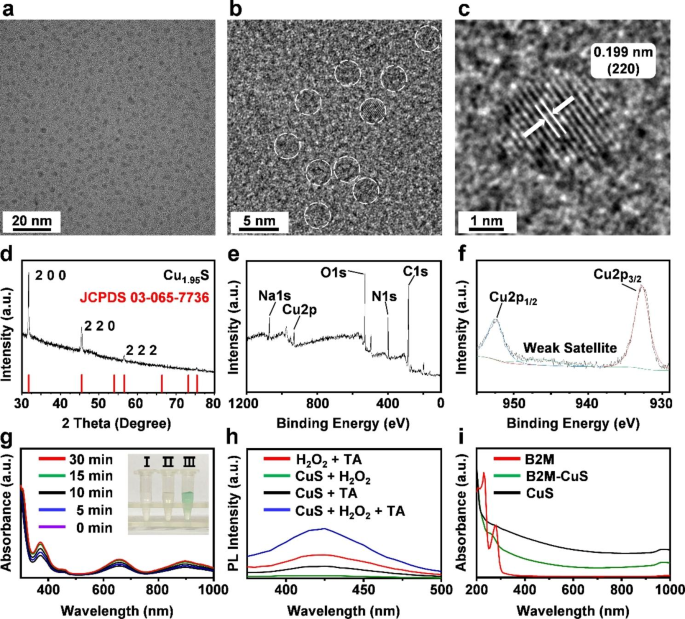
Characterization and enzyme-like exercise of NPs. (a, b) HRTEM photographs of CuS. NPs are highlighted with white dotted strains. (c) The lattice spacing of CuS NPs. (d) XRD sample of the synthesized CuS NPs corresponds with the usual Cu1.95 S crystal phases. (e, f) XPS spectra of CuS NPs. (g) Peroxidase-like actions of CuS NPs at totally different time intervals (0, 5, 10, 15, and 30 min). Inset: (I) TMB + H2O2, (II) TMB + CuS NPs, and (III) TMB + H2O2 + CuS NPs. The answer of CuS NPs + H2O2 + TMB generates blue response merchandise from the oxidation of TMB, indicating the peroxidase-like exercise of CuS NPs. (h) Fluorescence spectra after the response of CuS, TA, and H2O2, demonstrating that CuS NPs catalyze ∙OH formation from H2O2. (i) Absorption spectra of B2M antibody, CuS, and B2M-CuS NPs.
Institution of senescent chondrocytes
The mouse embryonic carcinoma-derived chondrogenic cell line ATDC5 was used for in vitro research. ATDC5 cells have been cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; VivaCell, Shanghai, China) and 1% penicillin/streptomycin at 37 ℃ beneath 5% CO2. Senescent chondrocytes have been established by Dox remedy in keeping with earlier studies [32, 33]. Briefly, ATDC5 cells have been handled twice with 0.1 µM Dox (48 h remedy interval), and senescent chondrocytes have been established after 4 days of stimulation.
Immunofluorescence staining of senescent cells
Regular and DOX-induced senescent ATDC5 cells have been fastened with 4% paraformaldehyde for 15 min, permeabilized with phosphate-buffered saline (PBS) containing 0.25% Triton X-100 for 10 min, after which blocked with 4% (v/v) BSA for 30 min at RT. Cells have been then incubated with main antibody in a single day at 4 ℃, washed thrice with PBS, after which incubated with the corresponding secondary antibody for two h. After washing with PBS, cell nuclei have been stained with 4,6-diamino-2-phenyl indole (DAPI) for five min, after which cells have been imaged by fluorescence microscopy.
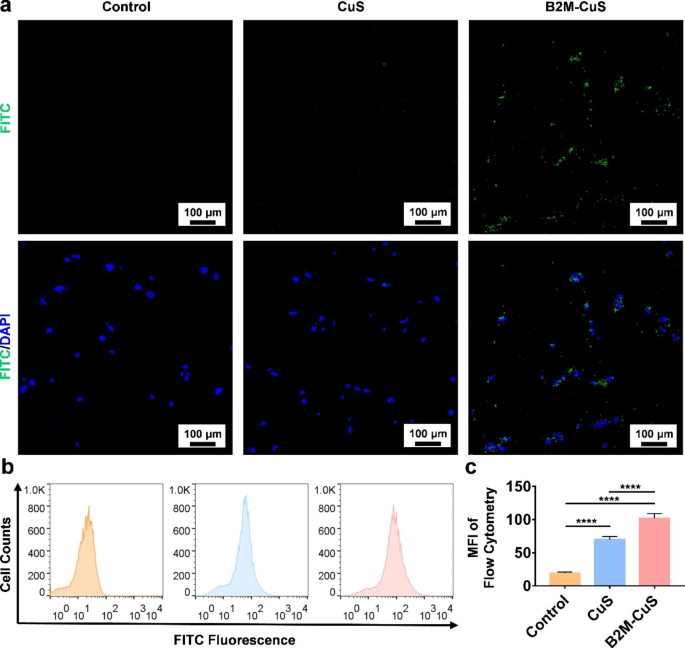
Concentrating on of senescent cells by B2M-CuS NPs. (a) Consultant fluorescent photographs of senescent chondrocytes co-cultured with FITC-labeled CuS or B2M-CuS NPs. Stronger inexperienced fluorescence (the FITC-labeled NPs) was noticed within the B2M-CuS group relative to the CuS group. Nuclei have been stained with DAPI (blue fluorescence). (b) Consultant circulation cytometry photographs of FITC fluorescence evaluation in senescent cells co-cultured with NPs. (c) Quantitative evaluation of the imply fluorescence depth of FITC calculated from (b). Information are introduced as imply ± SD (n = 3). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
SA-β-galactosidase staining
To measure the marker senescence-associated β-galactosidase (SA-β-gal) [34, 35], each regular and senescent ATDC5 cells have been stained utilizing a SA-β-gal staining package (Beyotime Institute of Biotechnology, Nanjing, China) in keeping with the producer’s directions. Cells have been washed with PBS, fastened with paraformaldehyde for 15 min at RT, after which stained in a single day at 37 ℃. Photographs have been acquired utilizing a microscope.
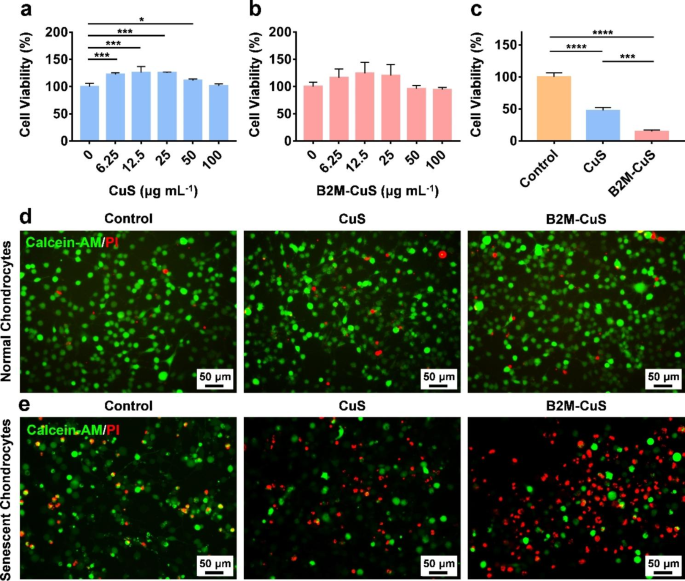
Cytotoxicity of CuS NPs towards regular and senescent chondrocytes. (a, b) Cell viability of regular chondrocytes co-cultured with CuS and B2M-CuS NPs at numerous concentrations for twenty-four h. (c) Cell viability of senescent chondrocytes co-cultured with CuS or B2M-CuS NPs (50 µg mL− 1) for twenty-four h. Stay/lifeless staining of regular chondrocytes (d) and senescent chondrocytes (e) co-cultured with CuS NPs or B2M-CuS NPs (50 µg mL− 1) for twenty-four h. Information are introduced as imply ± SD (n = 3). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
Cytotoxicity of the NPs
The cytotoxicity of CuS and B2M-CuS NPs was investigated by the usual CCK-8 methodology. Regular ATDC5 cells have been seeded in a 96-well plate (1 × 104 cells per nicely) and cultured with DMEM containing 10% FBS for twenty-four h at 37 ℃. Cells have been then cultured with CuS and B2M-CuS NPs at a number of concentrations (100, 50, 25, and 10 µg mL− 1) for twenty-four h. Thereafter, cells have been incubated with 10% CCK-8 answer for an extra 2 h and optical density (OD) values have been measured at 450 nm utilizing a microplate reader.
Hemocompatibility of NPs
The hemocompatibility of CuS and B2M-CuS NPs was evaluated. Purple blood cells (RBCs) derived from wholesome C57BL/6 mouse blood have been diluted with PBS (pH 7.4, 4:5 v/v). The NPs have been suspended in PBS and incubated at 37 ℃ for 30 min. After incubation, 200 µL of diluted RBCs have been added to the samples and incubated at 37 ℃ for an extra 1 h. PBS and ddH2O have been unfavorable and constructive management samples, respectively. After centrifugation at 3000 rpm for five min, the supernatant OD545 values have been measured on a microplate reader. The hemolysis ratio was calculated by the next components: Hemolysis ratio (%) = (ODpattern – ODunfavorable) / (ODconstructive – ODunfavorable) × 100.
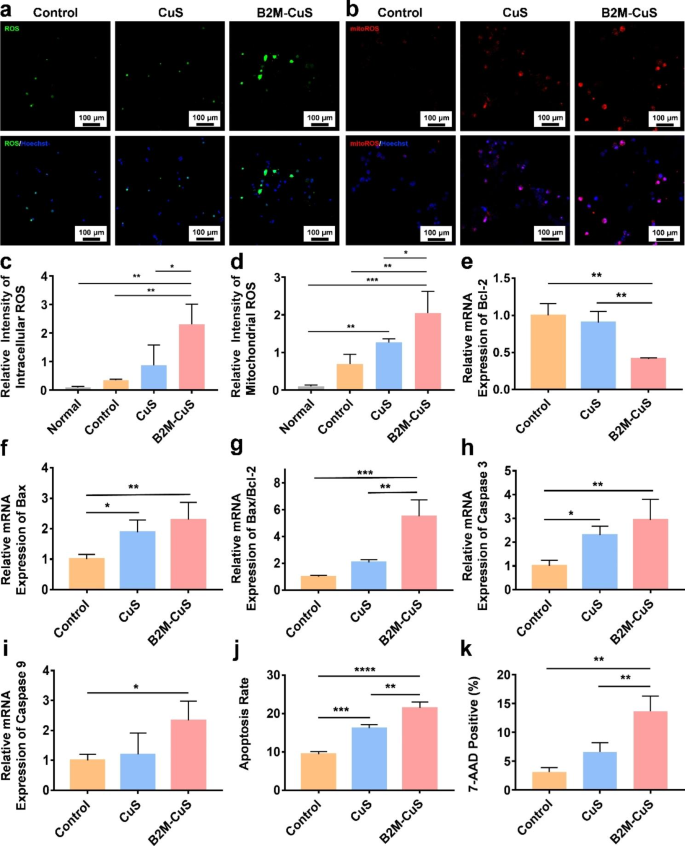
Apoptosis of senescent chondrocytes induced by B2M-CuS NPs. (a) Consultant fluorescence photographs of whole intracellular ROS in senescent chondrocytes after co-culturing with NPs (50 µg mL− 1) for twenty-four h. (b) Consultant fluorescence photographs of mitochondrial ROS in senescent chondrocytes following remedy. (c) Quantitative evaluation of the relative fluorescence depth of whole ROS derived from (a). (d) Quantitative evaluation of the relative fluorescence depth of mitochondrial ROS derived from (b). (e-i) Relative mRNA expression of Bcl-2, Bax, Bax/Bcl-2, Caspase-3, and Caspase-9 in senescent chondrocytes following remedy (n = 3). (j) Quantitative evaluation of apoptosis in senescent chondrocytes following remedy. (ok) Quantitative evaluation of 7-AAD constructive cells in numerous teams. Information are introduced as imply ± SD (n = 3). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
Concentrating on impact of B2M-CuS NPs on senescent chondrocytes
To organize FITC-labeled NPs, 5 µL of FITC-xtra answer (20 mM) was added to five mL of CuS and B2M-CuS NPs options (5 mg). After stirring on ice for 60 min, free FITC-xtra was eliminated by centrifugation for five min at 10,000 rpm.
To research the concentrating on specificity of B2M-CuS NPs, senescent ATDC5 cells and regular ATDC5 have been seeded in a 48-well cell tradition plate and grown for twenty-four h. Subsequently, FITC-labeled CuS and B2M-CuS NPs (50 µg mL− 1) have been added and cultured with cells for an extra 4 h. After eradicating the supernatant, cells have been washed with PBS, fastened with 4% paraformaldehyde for 15 min, stained with DAPI for five min, and visualized beneath a confocal laser scanning microscope (LSCM; Nikon ECLIPSE Ti2-E, Japan). As a management, senescent cells have been first handled with free B2M antibody for six h, after which B2M-CuS NPs have been added. After 24 h, cells have been washed and noticed. Moreover, circulation cytometry was carried out to detect the fluorescence depth of handled senescent cells.
Particular elimination of senescent chondrocytes by NPs
To check the precise elimination of senescent chondrocytes by the NPs, DOX-induced senescent ATDC5 cells have been seeded on a 48-well cell tradition plate (5 × 104 cells per nicely). CuS and B2M-CuS NPs (50 µg mL− 1) have been added to the plate and cultured with cells for twenty-four h, and cell viability was assayed with CCK-8. Stay/lifeless staining was additionally carried out. The above-treated cells have been gently washed with PBS and stained with 200 µL of Calcein AM and propidium iodide (PI; BestBio, China) for 30 min at 37 ℃ at the hours of darkness. Cells have been imaged with a fluorescence microscope.
Detection of intracellular ROS and mitochondrial ROS
Senescent ATDC5 cells have been cultured with CuS and B2M-CuS NPs (50 µg mL− 1) for twenty-four h, and intracellular ROS was detected through a 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) methodology as we beforehand reported [36, 37]. Following remedy, cells have been washed with PBS after which incubated with 500 µL DCFH-DA working answer (20 µM) at 37 °C for 30 min. Nuclei have been Hoechst stained for five min. Mitochondrial ROS was detected by MitoROS™ 580 staining in keeping with the producer’s directions. MitoROS™ 580 working answer (200 µL) was added to every nicely and incubated for 10 min. Cells have been washed with PBS twice and noticed utilizing LSCM. As a reference, intracellular ROS and mitochondrial ROS ranges of regular chondrocytes have been measured.
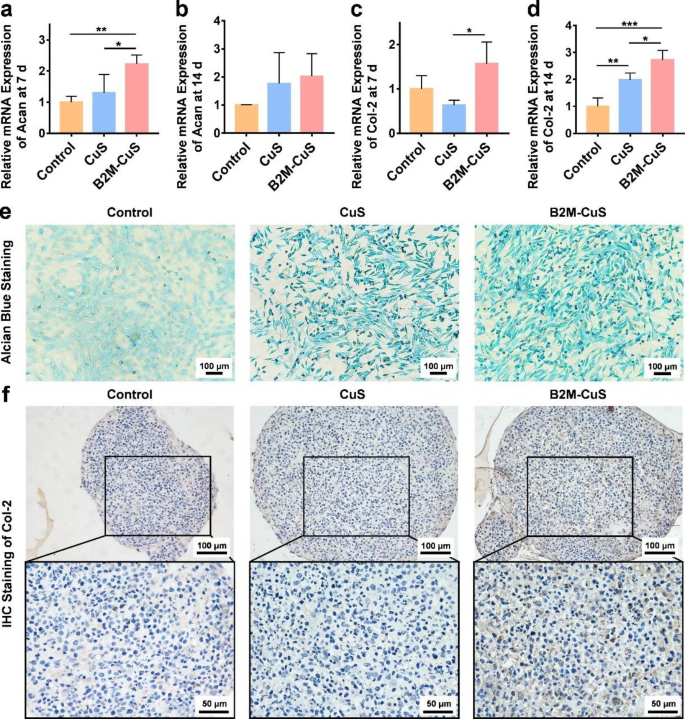
Chondro-inductivity of B2M-CuS NPs. (a-d) Relative mRNA expression of Acan and Col-2 in chondrocytes after co-culturing with NPs for 7 and 14 days. (e) GAG deposition (visualized by alcian blue staining; blue shade) in chondrocytes after co-culturing with NPs for 7 days. (f) Immunohistochemistry staining of Col-2 (brown shade) in cell pellets co-cultured with NPs for 21 days. Information are introduced as imply ± SD (n = 3). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
Apoptosis and necrosis of senescent chondrocytes
After culturing with CuS and B2M-CuS NPs (50 µg mL− 1) for twenty-four h, senescent ATDC5 cells have been collected and incubated with 100 µL of Annexin V-FITC and PI answer (BD, USA) for 20 min at RT. Cell apoptosis and necrosis have been measured by circulation cytometry and the outcomes have been analyzed with FlowJo X. To research the elimination of senescent cells by NPs, handled cells have been stained with 7-aminoactinomycin D (7-AAD) for 30 min after which analyzed by circulation cytometry.
The expression ranges of apoptosis-related genes (e.g., Bax, Bcl-2, caspase-3, and caspase-9) have been decided by real-time quantitative PCR (RT-qPCR). Complete RNA from handled senescent ATDC5 cells was extracted with Trizol (Thermo Fisher Scientific), and RNA concentrations have been decided by spectrophotometry (Nanodrop 2000; Thermo Fisher Scientific). RNA was reversed transcribed into cDNA utilizing the Evo M-MLV reagent package in keeping with the producer’s directions. The biking protocol was: 95 ℃ for 15 min, adopted by 45 cycles of 95 ℃ for five s and 60 ℃ for 30 s. The outcomes have been analyzed with Rotor-Gene Actual-Time software program. Relative mRNA expression ranges of every gene have been normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ranges utilizing the two−ΔΔCT methodology. Primers used for RT-qPCR are listed in Desk S1.
Chondrogenic inductivity of the NPs
To guage the in vitro chondrogenesis induced by NPs, ATDC5 cells have been cultured with chondrogenic medium (MUXMX-90,041, Cyagen Bioscience, USA) containing CuS NPs or B2M-CuS NPs (50 µg mL− 1). After 7 days, cells have been washed with PBS, fastened with 4% paraformaldehyde for 15 min, and stained with alcian blue 8GX answer for 30 min at RT to detect intracellular glycosaminoglycan (GAG). After washing with PBS thrice, cells have been noticed with a microscope.
To find out the expression stage of chondrogenesis-related genes aggrecan (Acan) and Col-2, cells have been collected after 7 and 14 days of chondrogenic induction and RT-qPCR was carried out.
As well as, ATDC5 cells have been induced into cell pellets, as beforehand reported, and cultured in chondrogenic medium with NPs (50 µg mL− 1) [38]. The medium was modified each 3 days. After 21 days, cell pellets have been collected and glued in 4% paraformaldehyde, embedded in paraffin, and lower into sections. Col-2 expression was detected by immunohistochemistry.
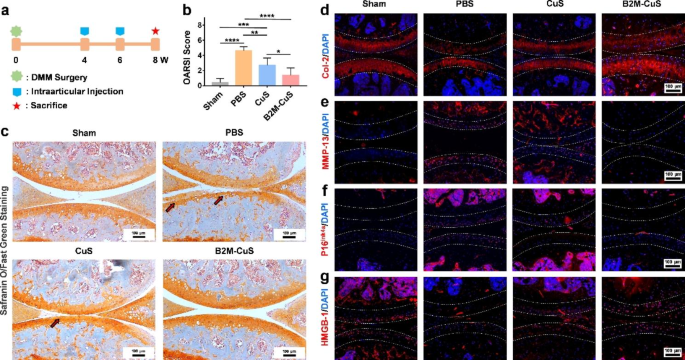
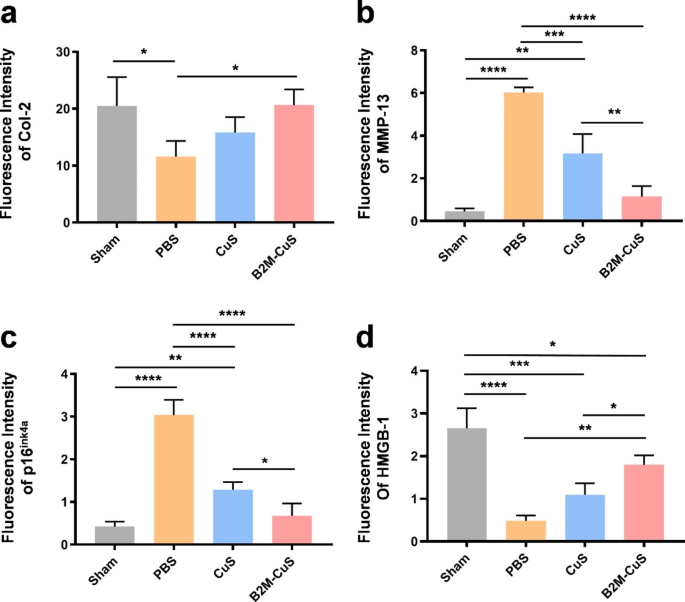
Therapeutic results of B2M-CuS NPs in a surgery-induced OA mouse mannequin. (a) Schematic diagram of the experimental design. B2M-CuS NPs, CuS NPs, and PBS have been intra-articularly injected into the knee joints of OA mice each 2 weeks starting at 4 weeks post-surgery. Knee joints have been collected at 8 weeks post-surgery. (b) OARSI scores have been measured from histological sections. (c) Consultant photographs of Safranin O/quick inexperienced staining of the knee joints after remedy. Cartilage lesions are highlighted by pink arrows. (d–g) Immunofluorescence staining of knee joints at 8 weeks post-surgery. The expression of Col-2 (d) was increased whereas the expression of MMP-13 (e) was decrease within the B2M-CuS group relative to the CuS and PBS teams. Senescence marker p16ink4a(f) was extremely expressed whereas HMGB-1 expression (g) was negligible within the PBS group. The expression of p16ink4a and HMGB-1 have been comparable between B2M-CuS and sham teams, indicating that B2M-CuS NPs successfully eradicated senescent chondrocytes. Cartilage areas are highlighted by white dotted strains. Information are introduced as imply ± SD (n = 6). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
Therapeutic results of the NPs in an OA mouse mannequin
All animal research have been accredited by the Institutional Animal Care and Use Committee (IACUC) of the Normal Hospital of Southern Theater Command of PLA (2021032401). Grownup male C57BL/6 mice (20 g, 12 weeks) have been anesthetized and induced into surgical destabilization of the medial meniscus (DMM) [38]. Briefly, the medial meniscus supporting ligament (MMSL) was resected after a medial capsular incision was made on the suitable knee joint of mice. After 4 weeks, OA mice fashions have been established. The management group acquired sham surgical procedure (opening of the joint capsule with out MMSL resection; n = 6).
The OA mice have been randomly divided into three remedy teams (n = 6 per group): (1) PBS, (2) CuS NPs, and (3) B2M-CuS NPs. The NPs (10 µL, 2.5 mg kg− 1) and PBS (10 µL) have been delivered by intra-articular injection into the suitable knee of the OA mice at 4 weeks and 6 weeks post-surgery, respectively. Mice have been sacrificed at 8 weeks post-surgery and the suitable knee joints have been collected for additional analysis.
Histological evaluation and biodistribution
For histological evaluation, the suitable knee joint samples have been fastened in 4% paraformaldehyde in a single day, decalcified in an EDTA answer, and embedded in paraffin blocks. Sections have been lower into 5 μm-thick slices and stained with hematoxylin and eosin (H&E) and Safranin O/quick inexperienced. All sections have been visualized with an inverted microscope (Nikon TS100, Japan). The Osteoarthritis Analysis Society Worldwide (OARSI) scores have been evaluated in keeping with a earlier report [39]. Immunofluorescence staining for protein expression (Col-2, MMP-13, p16ink4a, and HMGB-1) was carried out following our beforehand reported strategies [38, 40]. Photographs have been obtained utilizing an inverted fluorescent microscope. The fluorescence depth was calculated utilizing ImageJ software program.
To guage the biodistribution of B2M-CuS NPs, mice have been intra-articularly injected with the NPs. After 24 h, mice have been sacrificed and the principle organs (coronary heart, liver, spleen, lung, kidney, and proper knee joint) have been collected and weighed. Samples have been digested with aqua regia and Cu2+ content material was decided by ICP-MS (Agilent 7700, Germany).
Statistical evaluation
Information are introduced as imply ± commonplace deviation (SD). Statistical comparisons amongst totally different teams have been carried out by utilizing one-way evaluation of variance (ANOVA) adopted by posthoc a number of comparisons (LSD take a look at). A p-value < 0.05 was thought-about statistically vital and a p-value < 0.01 was thought-about extremely statistically vital.