Profitable fabrication of extremely aligned nanofibers
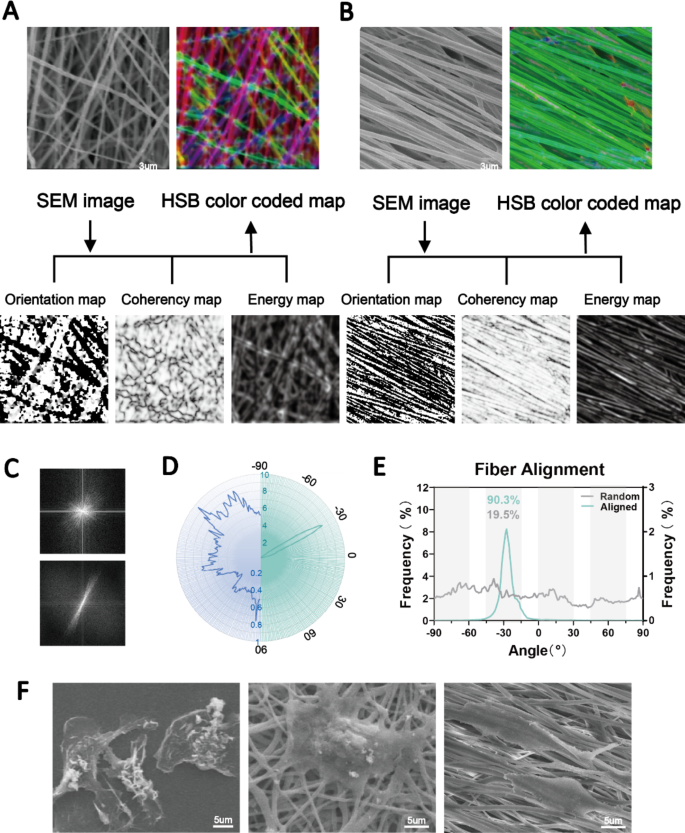
Excessive-speed rotation of the electrospun axial receiver permits for the technology of aligned fibers. Right here, we ready extremely aligned PCL fibers in distinction to random fibers. The SEM photos confirmed that each the aligned and random nanofibers had easy surfaces and had been uniform in diameter. We evaluated the orientation of the fibers based mostly on the construction tensors utilizing Orientation J [24]. Native orientation was analyzed and is proven in Fig. 2A. To successfully embrace solely the angular values of the fiber edges, we utilized the isotropic properties (coherency and vitality) to differentiate between the uniform and edge areas. The colour-coded maps are composites of the orientation, coherency and brightness maps, and so they point out the oriented constructions of the fibers. Moreover, we quantified the orientation of the aligned and random fibers (Fig. 2B-D), which exhibited considerably completely different patterns of angle distribution. A complete of 90.3% of the aligned fibers had been oriented from − 45° to − 15°, whereas the random fibers had been oriented in a nonfocused method (Fig. 2B). The Fourier evaluation and radar charts confirmed related outcomes, the place the orientation of the aligned fibers was in focus (Fig. 2C, D, E).
Fabrication of aligned and disordered electrospun nanofibers. (A, B) SEM photos and the orientation, vitality, coherency and HSB color-coded maps of aligned and random PCL nanofibers. (C, D, E) Dedication of the orientations of aligned and random fibers as visualized by Fourier and radar charts. (F) SEM photos displaying macrophages completely different morphologies hooked up to TCP substrates (left panel), random nanofibers (center panel) and aligned nanofibers (proper panel)
Subsequently, we plated bone marrow-derived macrophages on the 2 fibers and on TCP as a management. The outcomes confirmed that the fiber orientation considerably affected the extension of the cells. We noticed macrophages with an elongated morphology on the aligned fibers and polygonal macrophages on the random fibers (Fig. 2F). These outcomes indicated the profitable preparation of extremely aligned nanofibers. Angelina et al. reported that aligned PCL fibers extra strongly induced M2 macrophage polarization than random fibers [17]. Nonetheless, inflammatory reactions to overseas our bodies had been nonetheless inevitable. Subsequently, this research targeted on additional enhancing the physicochemical properties of the fibers to realize higher immune tolerance and regulation. To unify the variables, solely aligned fibers had been used for in vitro and in vivo evaluation.
Characterization of hydrophilic nanofibers with an aligned topography
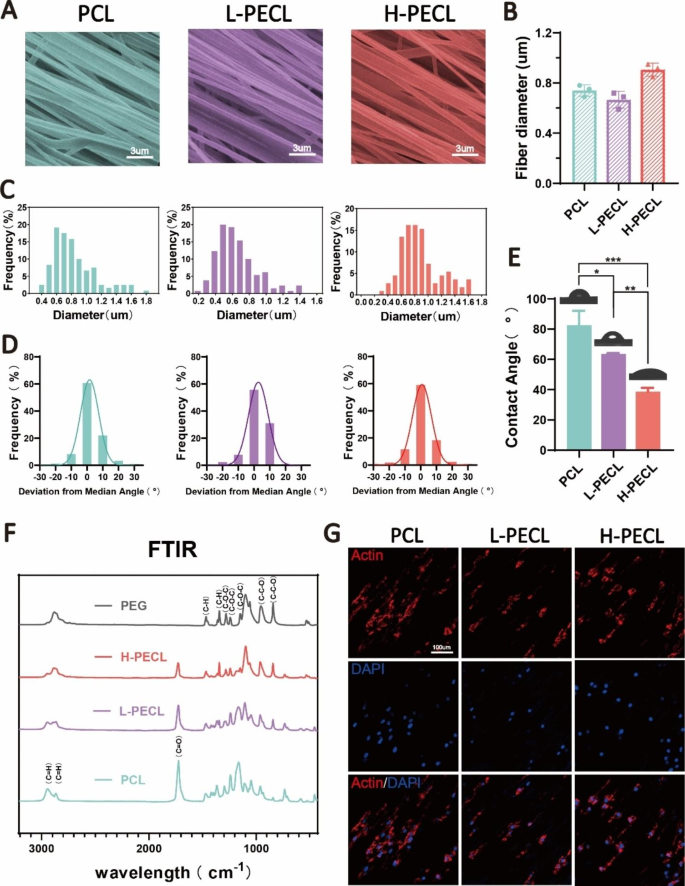
To change the floor properties of the nanofibers, we ready aligned nanofibers by incorporating PCL and PEG by axial electrospinning. H-PECL and L-PECL check with the nanofibers with excessive and low PEG proportions, respectively, which had completely different levels of hydrophilicity [25, 26]. The SEM photos confirmed that the nanofibers in every group had been comparatively uniform in dimension, with easy surfaces and no bead-like constructions. Moreover, the nanofiber movies had been extremely aligned (Fig. 3A). The diameters of the PCL, L-PECL, and H-PECL fibers had been 0.74 ± 0.05 μm, 0.66 ± 0.07 μm, and 0.91 ± 0.05 μm, respectively (Fig. 3B, C). The H-PECL fibers had a bigger diameter. We analyzed the orientation of the three sorts of nanofibers. The deviation angles had been largely concentrated within the vary of -15° to fifteen° (Fig. 3D). This indicated that all the membrane sorts had been effectively oriented. Then, we measured their water contact angles (Fig. 3E), and the outcomes advised that the incorporation of PEG elevated the hydrophilicity of the fibers. Furthermore, the hydrophilicity elevated with growing PEG proportion. The chemical composition of the nanofibers was characterised by FTIR. With growing proportions of PEG, the depth of the C = O stretching band of PCL decreased, whereas the depth of the C-O-C stretching band of PEG elevated. The L-PECL and H-PECL fibers exhibited traits of each PCL and PEG (Fig. 3F).
Characterization of PEG-modified aligned fibers with completely different levels of hydrophilicity. (A) SEM photos, (B) diameter distribution and (C) common diameters of the three sorts of nanofibrous membranes (from left to proper PCL, L-PECL, and H-PECL). (D) Dedication of the orientations and (E) water contact angles of the various kinds of membranes (n = 3). (F) Fourier remodel infrared (FTIR) spectra of the PCL, L-PECL, and H-PECL nanofibers. (G) Cytoskeleton staining displaying the oriented extension of macrophages alongside the nanofibers (Actin: purple and nucleus: blue). The info are offered because the imply ± normal deviation of three experimental replicates. Statistical evaluation was carried out by one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance
To analyze the impact of nanofibers on macrophages, we cultured BMDMs on the three sorts of fibrous nanomembranes. We noticed that the BMDM adhered to the fibers, and the macrophages prolonged alongside the course of the fibers (Fig. 3G). This advised that the nanofibers considerably affected the morphology of the macrophages. We then assessed the adhesion of macrophages to completely different nanofiber membranes by analyzing the formation of stress fibers. Normally, the semiquantitative evaluation of the stress fiber formation confirmed a lower in stress fibers as the quantity of PEG elevated, as proven in Determine S1. This hole could also be attributed to the non-adherent nature of PEG to proteins or cells.
Nanofiber hydrophilicity alters the phenotype of macrophages
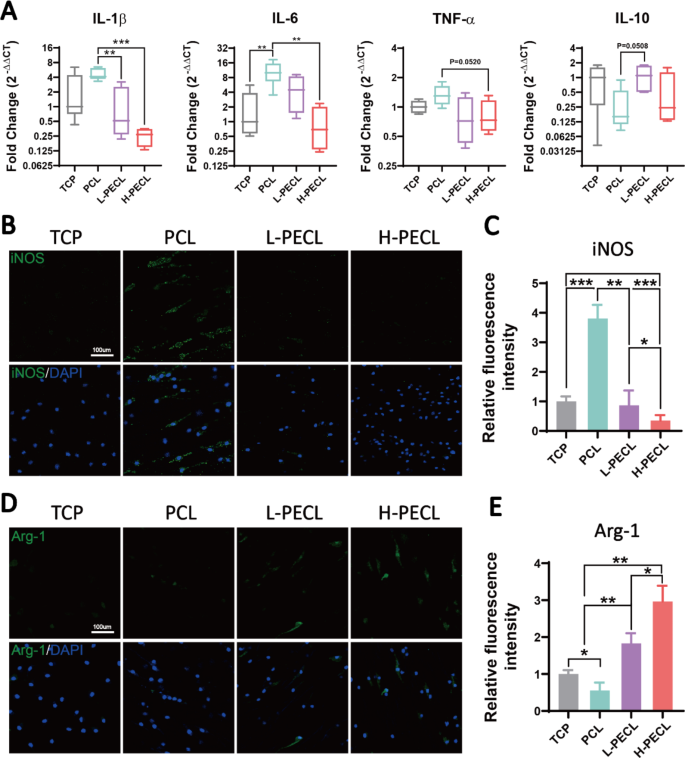
We seeded BMDMs on every kind of fibrous membrane to investigate the adjustments in macrophage phenotype. First, we measured the expression ranges of inflammatory genes. In comparison with the macrophages on the TCP management, the macrophages on PCL nanofibers confirmed considerably increased expression of the proinflammatory genes IL-1β and IL-6 and decreased expression of the anti-inflammatory gene IL-10 (Fig. 4A). This confirmed that PCL induced an inflammatory response in macrophages. Because the proportion of PEG within the nanofibers elevated, the expression of inflammatory genes progressively decreased. Within the H-PECL group, the expression of IL-1β was even decrease than that within the TCP group. This can be because of the hydrophilic and axial properties of the nanofibers.
Modifications within the polarization of macrophages cultured on various kinds of nanofibers. (A) RT–PCR was used to find out the gene expression of the proinflammatory elements IL-1b, IL-6, and TNF-α and the anti-inflammatory issue IL-10 in BMDMs cultured on various kinds of nanofibers. (B, C) Immunofluorescence evaluation of iNOS, indicating M1 polarization (iNOS: inexperienced and nucleus: blue). (D, E) Immunofluorescence evaluation of Arg-1, indicating M2 polarization (Arg-1: purple and nucleus: blue). The info are offered because the imply ± normal deviation of three experimental replicates. Statistical evaluation was carried out by one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance
Subsequent, we analyzed the polarization of macrophages by immunofluorescence staining for the M1 phenotypic marker iNOS and the M2 phenotypic marker Arg-1. The outcomes confirmed outstanding optimistic staining for iNOS within the PCL group. In distinction, the expression of iNOS was decrease within the L-PECL and H-PECL teams (Fig. 4B, C). Arg-1 expression confirmed the alternative development, and the very best expression was noticed within the H-PECL group (Fig. 4D, E). These outcomes advised that PCL fibers induced macrophage polarization towards the proinflammatory M1 phenotype, whereas the incorporation of PEG lowered this shift and promoted macrophage polarization towards the anti-inflammatory M2 phenotype.
The NLRP3 inflammasome is the mobile sensor that’s accountable for hydrophilic nanofiber-driven macrophage polarization
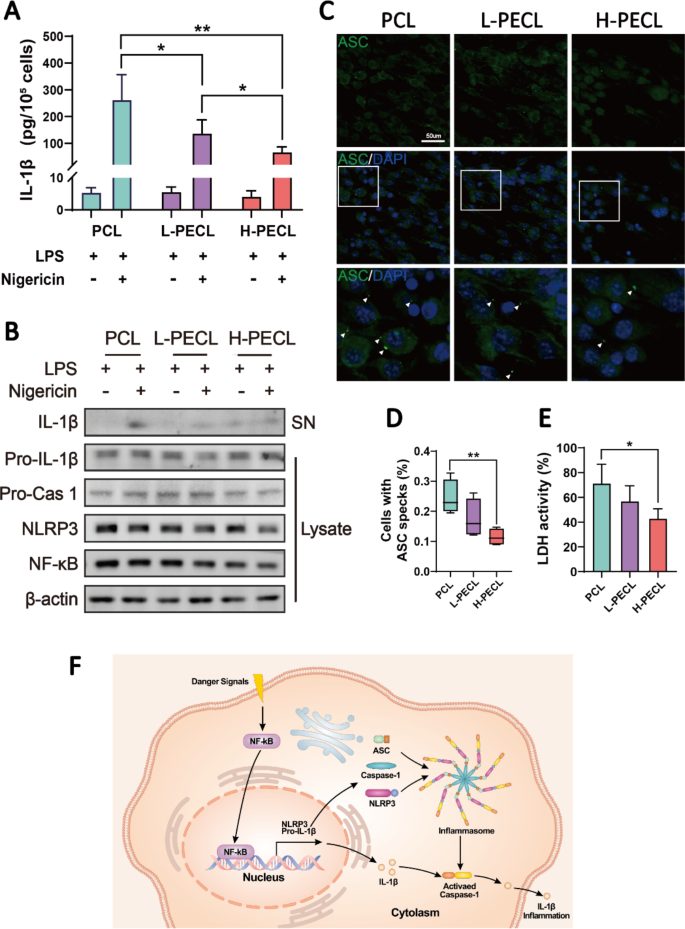
In line with the outcomes described above, we are able to infer that the polarization of macrophages differentiated after they had been cultured on various kinds of nanofibers. Accordingly, we proceeded to discover which pathways affected their polarization. From Fig. 4A, we noticed a considerable distinction within the expression of the proinflammatory gene IL-1β. Subsequently, we measured the secretion of IL-1β by BMDMs cultured on every kind of membrane by ELISA (Fig. 5A). Nonetheless, we discovered that IL-1β was expressed at low ranges in all of the teams. Contemplating that the secretion of IL-1β is carefully related to inflammasome activation, we stimulated BMDMs with LPS and nigericin and subsequently measured IL-1β secretion. The ELISA outcomes confirmed that extra IL-1β was launched by the macrophages in all teams after stimulation (Fig. 5A). Encouragingly, macrophages within the H-PECL group secreted much less IL-1β than these within the different two teams, and the distinction was statistically important.
IL-1b launch and NLRP3 inflammasome activation in macrophages cultured on various kinds of nanofibers. (A) ELISA was used to find out IL-1b launch by BMDMs stimulated with LPS and nigericin. (B) Western blotting evaluation of the expression of NLRP3 and associated signaling proteins in BMDMs stimulated with LPS and nigericin. (C) Immunofluorescence evaluation of ASC oligomerization in BMDMs (ASC: inexperienced and nucleus: blue), indicating inflammasome meeting. (D) Proportions of BMDMs with ASC specks. (E) Conceptual graphs of NLRP3 inflammasome activation and performance. The info are offered because the imply ± normal deviation of three experimental replicates. Statistical evaluation was carried out by two-tailed Scholar’s t take a look at and one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance
To additional examine the mobile sensor that’s accountable for nanofiber-driven macrophage polarization, we analyzed the expression of the NLRP3 inflammasome and associated proteins within the cytoplasm and supernatants of BMDMs. The outcomes confirmed decreased expression of NLRP3 and NF-kb within the macrophages cultured on nanofibers with PEG, and the degrees of IL-1b that had been secreted into the supernatants had been additionally lowered (Fig. 5B). Activation of the NLRP3 inflammasome includes the meeting of NLRP3, ASC and caspase-1 (Fig. 5F). Subsequently, we carried out extra immunofluorescence staining for ASC to visualise inflammasome meeting. Oligomerization of ASC was noticed in response to LPS and nigericin remedy, as indicated by the white arrows in Fig. 5C. As anticipated, the variety of ASC specks within the macrophages within the H-PECL group was the bottom (Fig. 5D). Inflammasome meeting in macrophages results in pyroptosis. Consequently, lactate dehydrogenase (LDH) launch by macrophages signifies the diploma of pyroptosis [27]. The macrophages cultured on PEG-modified nanofibers exhibited decrease ranges of LDH exercise, indicating fewer pyroptotic cells (Fig. 5E). These outcomes indicated that PEG modified the floor properties of the nanofibers and inhibited the activation of the NLRP3 inflammasome in macrophages.
Subcutaneous macrophage-mediated immune responses and inflammasome activation
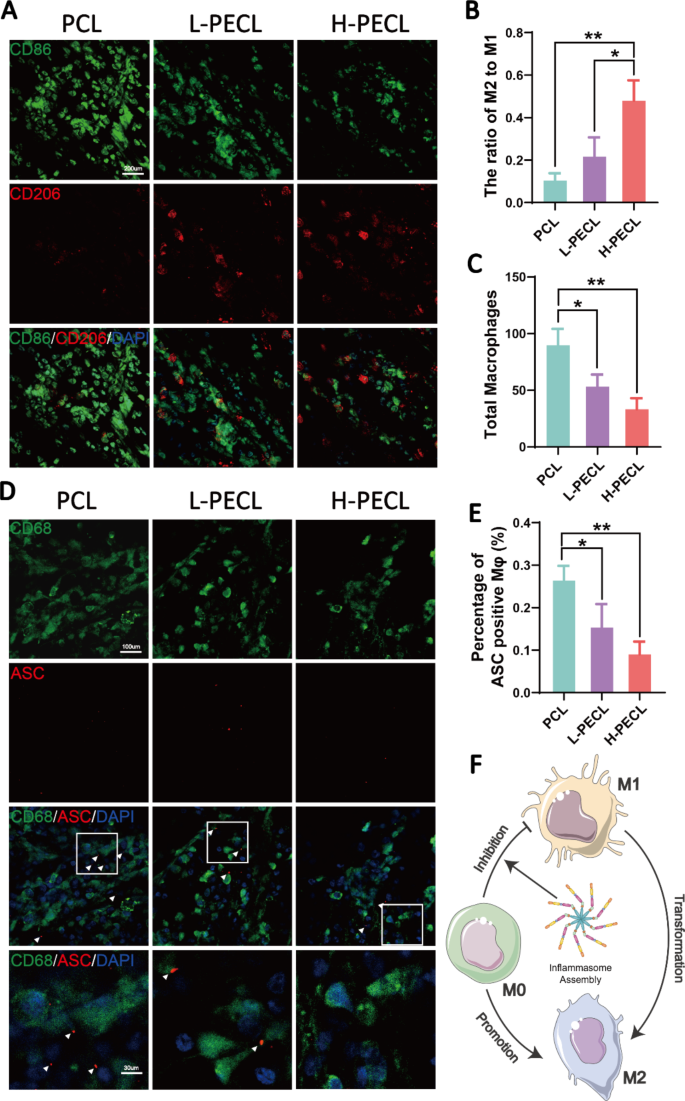
We subcutaneously implanted the three sorts of nanofibrous membranes into mannequin animals to discover the macrophage-mediated overseas physique response. Immunofluorescence staining was carried out to determine the macrophage phenotype. CD86 is a marker of M1 macrophages, whereas CD206 is a marker of M2 macrophages. As proven in Fig. 6, macrophage infiltration was noticed in all three teams, with the very best infiltration within the PCL group (Fig. 6A, C). In distinction, the H-PECL group had the bottom extent of macrophage infiltration and the very best proportion of M2-type macrophages (Fig. 6B, C). These outcomes advised that the PCL fibers elicited a stronger early host immune response. As well as, a lot of the macrophages that had been recruited to the nanofibrous membranes within the early stage exhibited a proinflammatory phenotype, whereas the incorporation of PEG into the nanofibers promoted macrophage polarization towards the anti-inflammatory phenotype. This helped to attenuate the overseas physique response and allow proactive immune regulation.
Subcutaneous macrophage recruitment, polarization and inflammasome activation. (A) Immunofluorescence evaluation of CD86 and CD206, indicating macrophage recruitment and polarization, on various kinds of subcutaneous membranes (CD86: inexperienced, CD206: purple, and nucleus: blue). (B) Statistical evaluation of the ratios of M2 to M1 macrophages. (C) Statistical evaluation of the entire variety of recruited macrophages. (D) Immunofluorescence photos of subcutaneous ASC oligomerization in recruited macrophages (ASC: purple, CD68: inexperienced, and nucleus: blue). (E) Proportions of macrophages with ASC specks. (F) Conceptual graphs of macrophage polarization. The info are offered because the imply ± normal deviation of three experimental replicates. Statistical evaluation was carried out by one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance
Moreover, we carried out immunofluorescence staining for ASC to guage inflammasome activation in macrophages cultured on subcutaneously implanted nanofibers (Fig. 6D). CD68 is a pan macrophage marker. The outcomes confirmed that subcutaneously implanted nanofibers recruited macrophages and initiated the meeting of inflammasomes. The bottom share of ASC specks was noticed within the H-PECL group (Fig. 6E), suggesting that PEG-modified nanofibers may scale back irritation by inhibiting inflammasome meeting throughout the early phases of implantation (Fig. 6F).
In vivo research: PEG-incorporated aligned nanofibrous membranes mitigate peritendinous adhesions
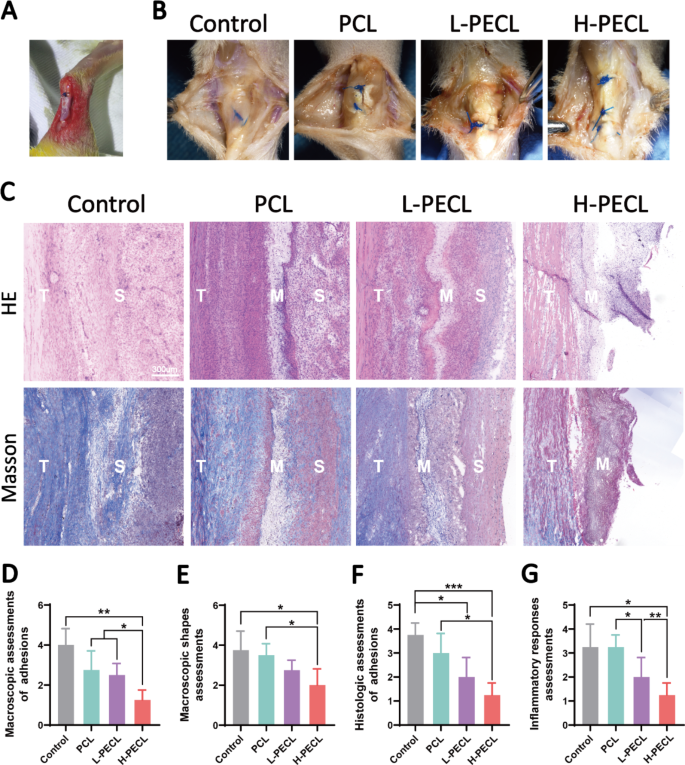
In line with the in vitro and subcutaneous experiments, we demonstrated that PEG-modified aligned nanofibers exhibited higher host compatibility and had been capable of scale back macrophage-mediated immune responses by lowering the expression of inflammatory genes and activation of the NLRP3 inflammasome. Subsequently, we thought of that the membranes might be used to forestall adhesion. For this function, we employed a rat mannequin of adhesion after Achilles tendon damage (Fig. 7A) and divided the animals into 4 teams: the management, PCL, L-PECL, and H-PECL teams. The animals had been sacrificed three weeks after surgical procedure, and peritendinous adhesions had been evaluated by gross commentary and tissue staining.
Macroscopic observations and histological staining of Achilles tendons handled with various kinds of nanofibers at 3 weeks post-surgery. (A) Surgical process by which the nanofibrous membranes had been wrapped across the Achilles tendon. (B) Macroscopic observations of tendons in every group. (C) H&E staining and Masson staining of the interface between the tendon and surrounding tissues. (D) Macroscopic assessments of adhesions. (E) Macroscopic form assessments. (F) Histological assessments of adhesions. (G) Histological assessments of the inflammatory response. The info are offered because the imply ± normal deviation from 4 experimental replicates. Statistical evaluation was carried out by one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance
As proven in Fig. 7B, extreme peritendinous adhesions developed after Achilles tendon damage. The adhesions had been lowered in all of the teams that had been handled with fibrous membranes. Nonetheless, substantial quantities of adherent tissue had been nonetheless noticed within the PCL group. In distinction, adhesions had been alleviated in each the L-PECL group and the H-PECL group, with simpler separation between the tendon and surrounding tissue. We scored the peritendinous adhesions macroscopically, and the H-PECL group had the bottom rating (Fig. 7D).
Subsequently, we carried out H&E and Masson staining (Fig. 7C). Dense fibrous tissue was noticed across the tendons within the management and PCL teams, which was in step with normal observations. The H-PECL group had the fewest adhesions, and the membranes appeared to have a sparse multilayer construction, which can be attributable to degradation in vivo. This contributed to the free gliding of the tendon. We carried out histological scoring of peritendinous adhesions and irritation (Fig. 7F, G), and the H-PECL group confirmed the most effective antiadhesive impact and the bottom inflammatory response.
Analysis of persistent inflammatory responses in response to the nanofibrous membranes
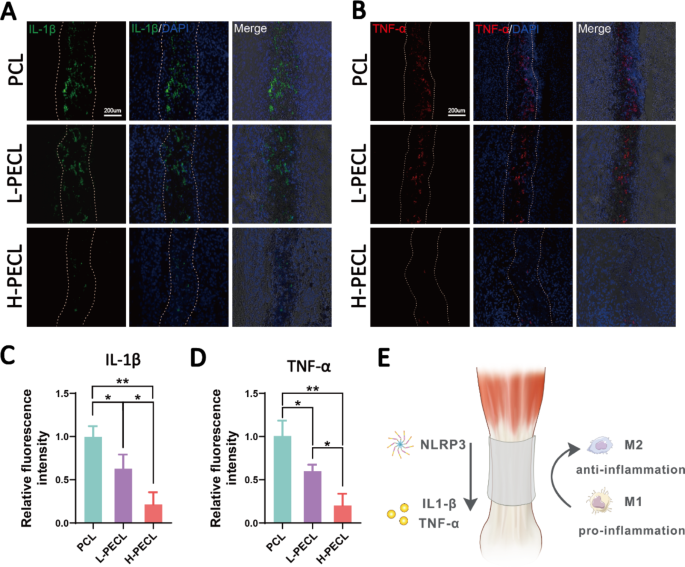
We carried out immunofluorescence staining for inflammatory elements to guage persistent inflammatory responses after implanting various kinds of nanofibrous membranes. The PCL group exhibited optimistic IL-1β and TNF-α staining, the L-PECL group exhibited weaker staining, and the H-PECL group had the weakest inflammatory issue expression (Fig. 8). This indicated that H-PECL nanofibers elicited a minimal inflammatory response after implantation and had been subsequently superior to PCL fibers when it comes to immune compatibility and modulation (Fig. 8D).
Analysis of inflammatory responses elicited by the nanofibers at 3 weeks post-surgery. Immunofluorescence evaluation of the inflammatory elements (A) IL-1b and (B) TNF-a within the implanted nanofibers. Fluorescence depth evaluation of (C) IL-1b and (D) TNF-α expression. (D) Conceptual graphs of the immune microenvironment of tendons. The info are offered because the imply ± normal deviation from 4 experimental replicates. Statistical evaluation was carried out by one-way ANOVA with Tukey’s submit hoc evaluation. *p < 0.05; **p < 0.01, ***p < 0.001; ns signifies no significance