In a research just lately printed within the journal Nanoscale, researchers from Kanazawa College and AGC Inc. use three-dimensional atomic power microscopy to check the hydrated kind and construction of generally occurring oxide crystals.
Whereas sapphire and quartz are oxide crystals utilized in a variety of business purposes, the atomic-scale constructions of those supplies are usually not nicely understood. The key chemical parts of sapphire and quartz are aluminum oxide and silicon dioxide, respectively. These parts have a excessive affinity for water, which impacts the chemical reactivity of the crystals. Thus, an intensive information of the water-binding properties of those oxides is necessary for additional revolutionary purposes.
Thus far, conventional microscopic strategies have solely offered insights into the two-dimensional topography of their surfaces. Now, a analysis crew led by Keisuke Miyazawa from the NanoLSI at Kanazawa College has developed three-dimensional (3D) microscopy method for an in depth research of the interplay of the surfaces of those supplies with water.
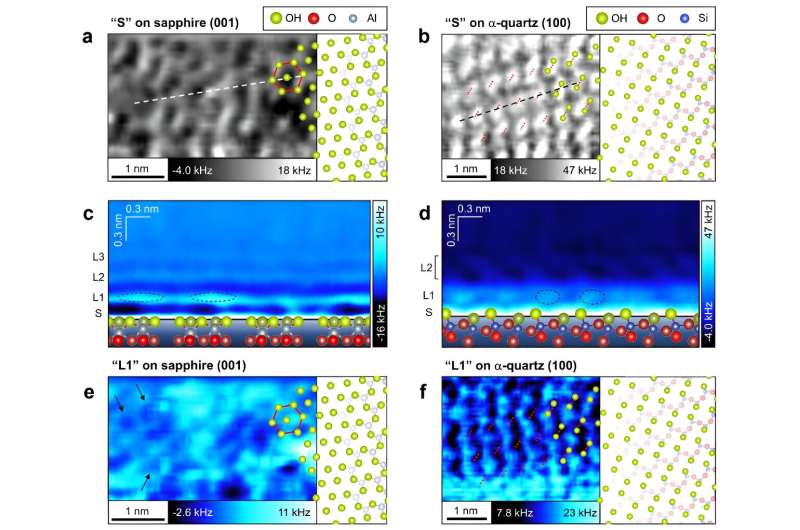
The crew began by trying on the floor constructions and its hydration constructions of sapphire and α-quartz in water. For this, they used a complicated type of microscopy often known as 3D atomic power microscopy (3D-AFM). Oxide crystals often have hydroxyl (OH) teams, that are the primary “water-binding” molecules, carefully linked with the oxides. Therefore, the crew studied the OH teams and its hydration constructions on each crystals when immersed in water.
They discovered that the hydration layer on sapphire was not uniform due to the nonuniform native distributions of the floor OH teams. Alternatively, the hydration layer on α-quartz was uniform due to the atomically flat distributions of the floor OH teams.
When the interplay power of those oxides for water was subsequently measured, it was discovered {that a} higher power was required to interrupt the water-crystal bonds in sapphire than in α-quartz. It was additionally found that this affinity was a lot larger in areas the place the oxides have been in shut proximity to the OH teams.
This research confirmed that the hydration constructions of oxides are depending on the situation and density of OH teams, along with the hydrogen bonding (the chemical bond used to bind to water) energy of the OH teams. What’s extra, it was efficiently proven right here that 3D-AFM can be utilized in unraveling the interplay of water with a number of surfaces, a possible avenue for understanding solid-liquid interactions higher.
“This research contributes to the appliance of 3D-AFM in exploring atomic scale hydration constructions on varied surfaces, and therefore, to a variety of stable–liquid interfacial analysis fields,” conclude the researchers.
3D atomic power microscopy (3D-AFM): AFM is a complicated type of microscopy whereby a pointy tip is mounted on a cantilever and follows the floor of a molecule. Because it does so, the tip emits alerts based mostly on its motion, which helps establish the topography of the molecule. Nonetheless, understanding the deeper constructions of molecules requires a three-dimensional overview of their surfaces. Thus, researchers used a extra superior model of AFM on this research, which captured the construction of hydrated crystals in 3D.
Extra data:
Sho Nagai et al, Three-dimensional ordering of water molecules reflecting hydroxyl teams on sapphire (001) and α-quartz (100) surfaces, Nanoscale (2023). DOI: 10.1039/D3NR02498A
Supplied by
Kanazawa College
Quotation:
Hydration issues: The interplay patterns of water and oxide crystals revealed (2023, September 11)
retrieved 18 September 2023
from https://phys.org/information/2023-09-hydration-interaction-patterns-oxide-crystals.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.

