Colour performs an enormous position in most of our lives. It indicators hazard or warning, just like the patterns on a toxic frog or the colour of a stoplight. It evokes pleasure and inspiration by nature, artwork, and vogue. It could possibly even set off the recollection of a favourite reminiscence, by photos of household and buddies.
Within the technical world, the colour of sunshine isn’t any much less necessary. It impacts the effectivity of photo voltaic cells, how far we are able to see inside our our bodies, and the velocity of 3-D printing.
However mild might be helpful provided that you possibly can truly get it the place it must go; many supplies will soak up or scatter the sunshine lengthy earlier than it might attain its meant vacation spot.
We within the
Congreve Lab at Stanford College grew to become serious about altering the colour of sunshine for precisely this cause: It could possibly assist us get the correct of sunshine to the fitting place.
Initially, we centered on creating color-changing expertise to be used in photo voltaic power, the place its usefulness is apparent. Photovoltaic cells harvest power from solely a restricted vary of energies—that’s, colours. That vary differs relying on the fabric used to supply the photo voltaic cell, nevertheless it’s all the time restricted. One method to enhancing solar-cell effectivity has been to supply cells with a number of layers tuned to completely different power ranges. However there may be one other method to consider it: It might be easier and extra environment friendly to alter the sunshine to suit the cell.
The Means of Upconversion
Earlier than we inform you extra about how this course of can increase solar energy in addition to revolutionize 3D printing and allow another thrilling functions, we’ll clarify how this expertise works.
Historically, the colour of a photon (outlined by its power or wavelength) is a given. But it seems that we are able to flip two low-energy photons right into a single higher-energy photon utilizing a course of referred to as upconversion.
Upconversion has been noticed in experiments for greater than 50 years, however low efficiencies saved it a laboratory curiosity till supplies that extra effectively emit upconverted mild have been found. Even then, points with extracting a sensible variety of upconverted photons, tips on how to incorporate the substances into the strong supplies wanted for real-life functions, and the supply of workable wavelengths have blocked upconversion’s path to commercialization.
These days, nevertheless, a flurry of effort from scientists all over the world has led to substantial
advances in every of these difficult areas. Most notably, researchers have found new supplies, made up of inorganic nanoparticles and metal-organic compounds, to extend the vary of enter and output wavelengths.
In our lab, we use these supplies to carry out a sort of upconversion course of referred to as triplet-triplet annihilation, which we’ll clarify in a second. There are different approaches that use the intrinsic skills of some uncommon
heavy components to conduct upconversion. However we selected the triplet-triplet annihilation path as a result of the enter energies required are low, so we don’t want costly pulsed lasers. As an alternative, we are able to use low-power lasers and even light-emitting diodes, whose depth is much like that of laser pointers. Simply as necessary, the supplies we’re utilizing are extra considerable. Collectively, these traits put our expertise on a better path to commercialization.
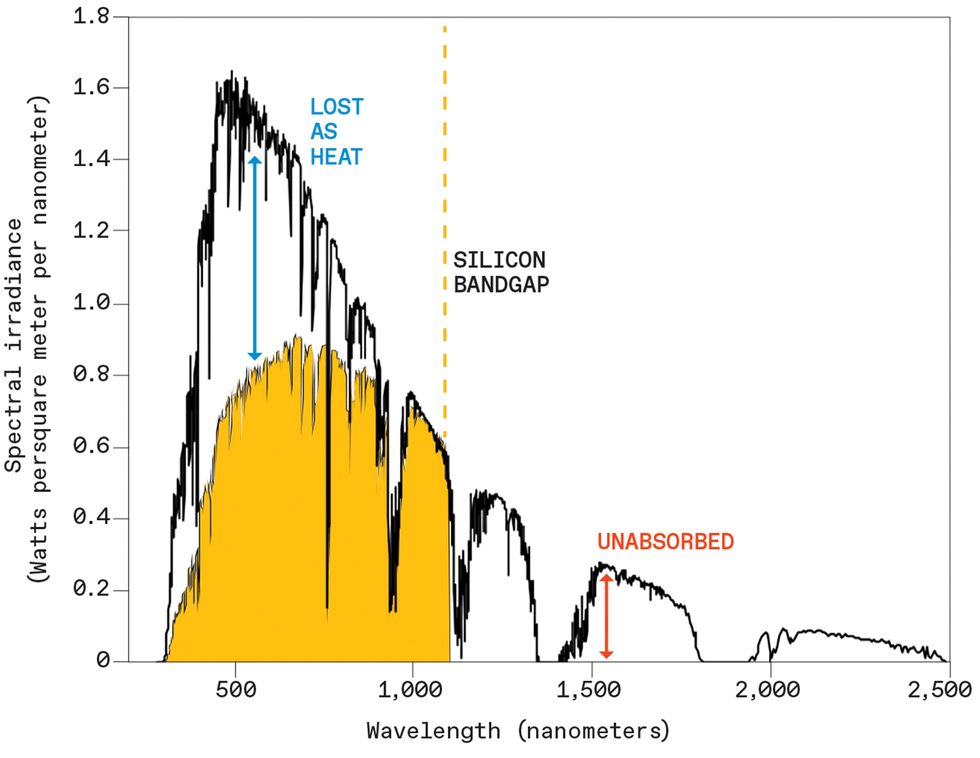 A silicon photo voltaic cell can effectively convert photons to electrical energy provided that the photons have energies near silicon’s bandgap. The cell loses a lot of the power from shorter-wavelength (higher-energy) photons as warmth, and it can’t soak up photons with decrease power. The authors are creating expertise that may convert a few of these unabsorbed wavelengths into colours near silicon’s candy spot.Supply: ASTM Worldwide
A silicon photo voltaic cell can effectively convert photons to electrical energy provided that the photons have energies near silicon’s bandgap. The cell loses a lot of the power from shorter-wavelength (higher-energy) photons as warmth, and it can’t soak up photons with decrease power. The authors are creating expertise that may convert a few of these unabsorbed wavelengths into colours near silicon’s candy spot.Supply: ASTM Worldwide
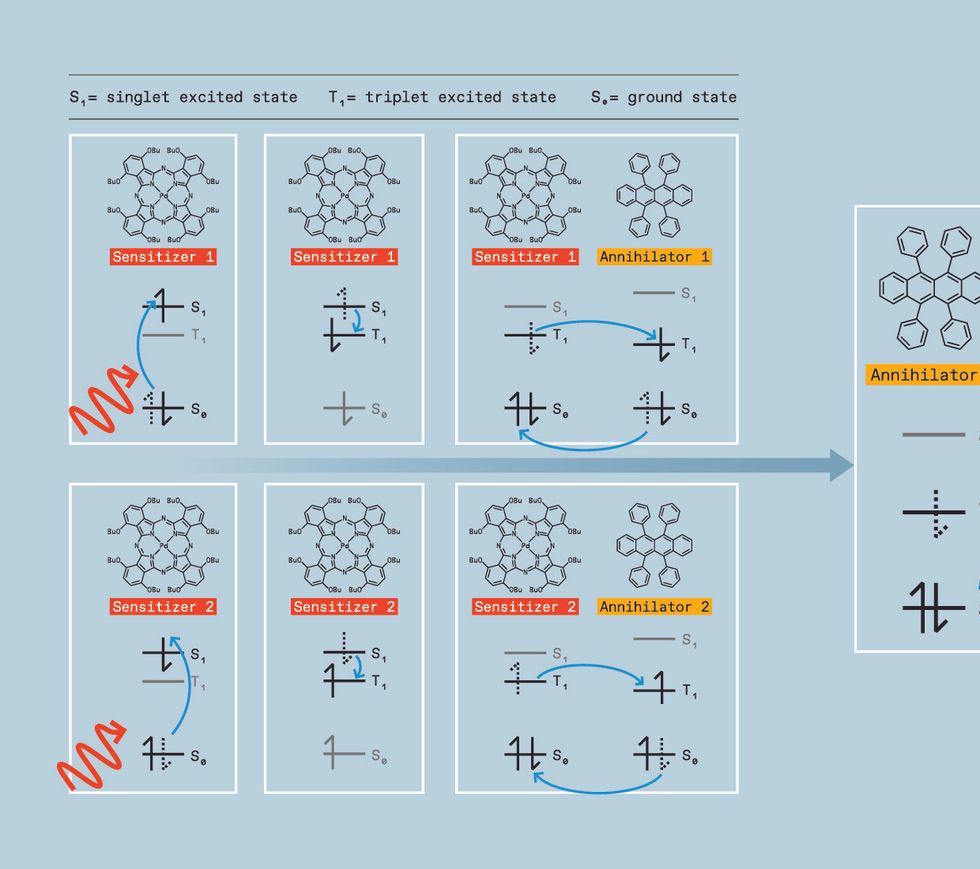
To know how triplet-triplet annihilation works, you first want to know the idea of electron spin states in natural molecules. Electrons in a molecule are organized in discrete areas. Consider the molecule as a multistory home. Every flooring has a single room representing one among these areas, or molecular orbitals. Every room can maintain two electrons, however they don’t make for good roommates until the electrons possess reverse qualities referred to as spin states. Electrons first fill the underside flooring—the bottom power areas—till all electrons have a room. If a photon hits this molecule (the home), it might excite one of many electrons to a better power state, pushing it to an unoccupied room on a better flooring. The electron stays there just for a pair nanoseconds or so earlier than it falls again to the bottom state—that’s, its unique room.
When an electron strikes again to the bottom state, the molecule releases the identical quantity of power—within the type of warmth or mild—that it absorbed to excite the electron. The short-lived excited state, during which the electron is up in its greater orbital, is named a singlet exciton.
There are different sorts of excitons. For instance, there’s a state during which the spins are unpaired (each spin up). Right here, as soon as one of many electrons is kicked as much as a better room, it can’t simply chill out again to its floor state as a result of that room is already occupied by an electron with the identical spin. Nonetheless, it does finally get there. Paint, stickers, and toys that glow in the dead of night after a interval of publicity to mild exploit this time lag.
Other than its use in novelty merchandise, this species, referred to as a triplet exciton, is normally considered as a nuisance. As an example, in natural light-emitting diodes (OLEDs), it’s the singlet excited states that emit mild. However each singlet and triplet excited states type in an OLED, with the triplet states lowering the sunshine we see and producing extra warmth, each belongings you don’t need in a show expertise.
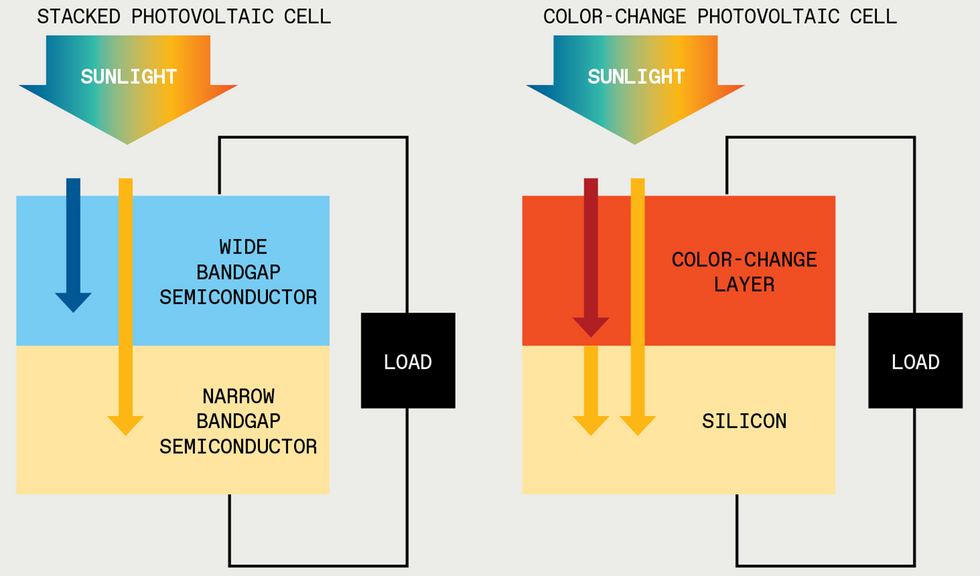 Daylight accommodates many wavelengths that silicon photo voltaic cells can’t use effectively. Quick wavelengths [blue arrow] can be absorbed, however their extra power is misplaced as warmth. And lengthy wavelengths [red arrow] aren’t absorbed in any respect. In the present day, researchers attempt to seize extra wavelengths by stacking a number of sorts of electricity-generating semiconductors atop each other, however this may be costly and tough to design. As an alternative, a layer of color-change materials might convert the lengthy wavelengths to colours that silicon can soak up, thereby simplifying the design and probably lowering the price.
Daylight accommodates many wavelengths that silicon photo voltaic cells can’t use effectively. Quick wavelengths [blue arrow] can be absorbed, however their extra power is misplaced as warmth. And lengthy wavelengths [red arrow] aren’t absorbed in any respect. In the present day, researchers attempt to seize extra wavelengths by stacking a number of sorts of electricity-generating semiconductors atop each other, however this may be costly and tough to design. As an alternative, a layer of color-change materials might convert the lengthy wavelengths to colours that silicon can soak up, thereby simplifying the design and probably lowering the price.
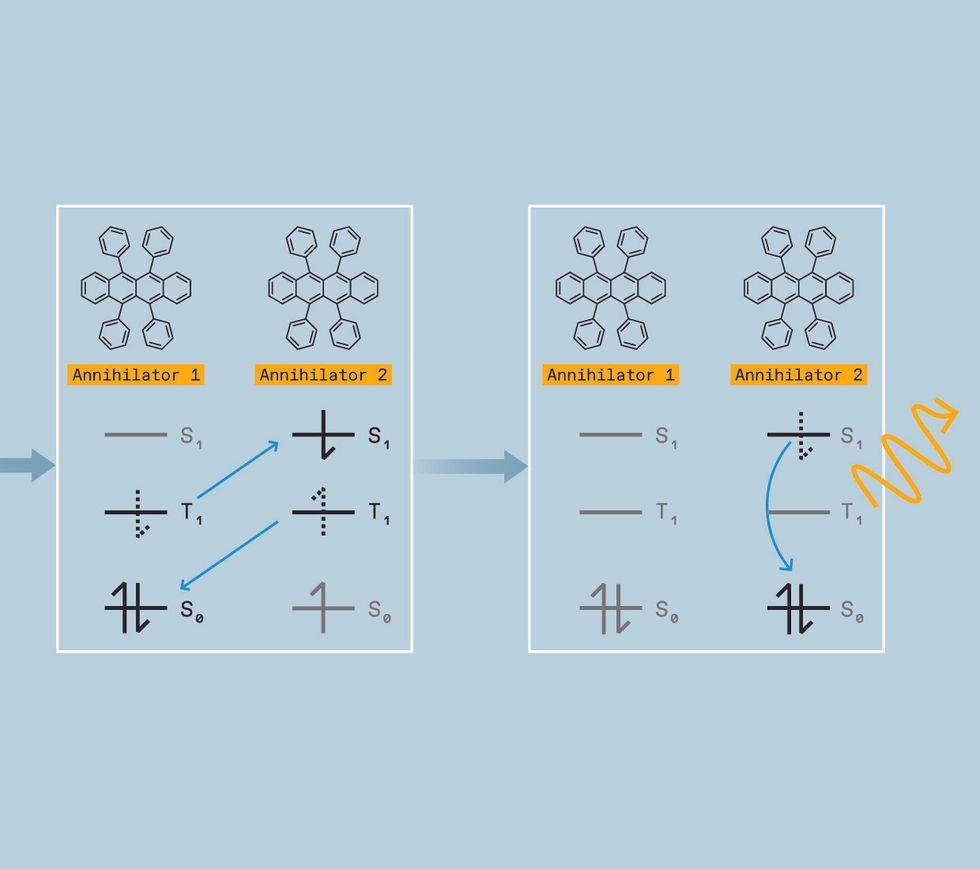
While you’re attempting to control the colour of sunshine, nevertheless, these triplet states have a silver lining. If two molecules in triplet states collide, they’ll generally mix their power. This course of is named triplet-triplet annihilation.
What pursuits us is that after combining, the ensuing molecule can emit a photon at a shorter wavelength—with greater power—simply as if the molecule had been excited with higher-energy mild immediately. Right here’s how we make that occur.
We begin by producing the triplet excited state, which is a problem. Whereas a number of courses of molecules, referred to as natural semiconductor annihilators, can enable triplets to mix, they don’t type triplets themselves when immediately hit with mild. As an alternative, we have to use a cloth referred to as a triplet sensitizer. Triplet sensitizers usually comprise a heavy steel like palladium, iridium, or platinum. This heavy steel serves as a mediator, making a path for the molecule to maneuver a singlet excited state to a lower-energy triplet excited state as an alternative of falling on to the bottom state.
The sensitizer can then donate its triplet to an annihilator molecule, which possesses a triplet excited state barely decrease in power than the sensitizer’s. When its power is transferred to the annihilator, the sensitizer returns to its floor state with out releasing mild. The annihilator molecule will finally emit mild—however not simply but.
To get the power launched as mild, we want two annihilators within the triplet excited state. So we hold pumping low-energy mild into the sensitizers, permitting them to repeat this course of again and again, producing a number of excited annihilators and growing the probabilities of two of those excited annihilators colliding.
When such a mash-up occurs, the annihilators switch power in a course of referred to as triplet-triplet annihilation, changing one molecule into the singlet excited state and the opposite molecule into the bottom state.
That singlet, nevertheless, has the mixed power of two triplets. So when it relaxes into the bottom state, the photon it emits is greater in power than the unique photon absorbed by the sensitizer. We now have upconverted two low-energy photons into one-high power photon. By way of colours, meaning we are able to take two purple photons and switch them right into a blue one, for instance, or take two infrared photons and switch them right into a yellow one.
Why Colour Issues in Photovoltaics
And that’s how we modify the colour of sunshine. Now to get again to the rationale we began doing this: photovoltaics.
Daylight presents considerable photons spanning a variety of energies, from the ultraviolet by the seen spectrum and into the infrared. But we use solely a fraction of the obtainable photons. That’s why a typical single-junction photo voltaic cell—a cell made of 1 layer of light-absorbing materials—has a theoretical effectivity restrict of simply 34 p.c; typical business photo voltaic cells as we speak are solely 15 to twenty p.c environment friendly. The only largest supply of this loss is a mismatch between the colours of incoming mild and the colours of sunshine that can be utilized by a photo voltaic cell.
To know this example, keep in mind that photovoltaic cells are manufactured from semiconductors, supplies that possess an power bandgap. When power is utilized, electrons will transfer from the valence band (the bottom state) to the conduction band (the excited state) and might be harnessed as electrical power.
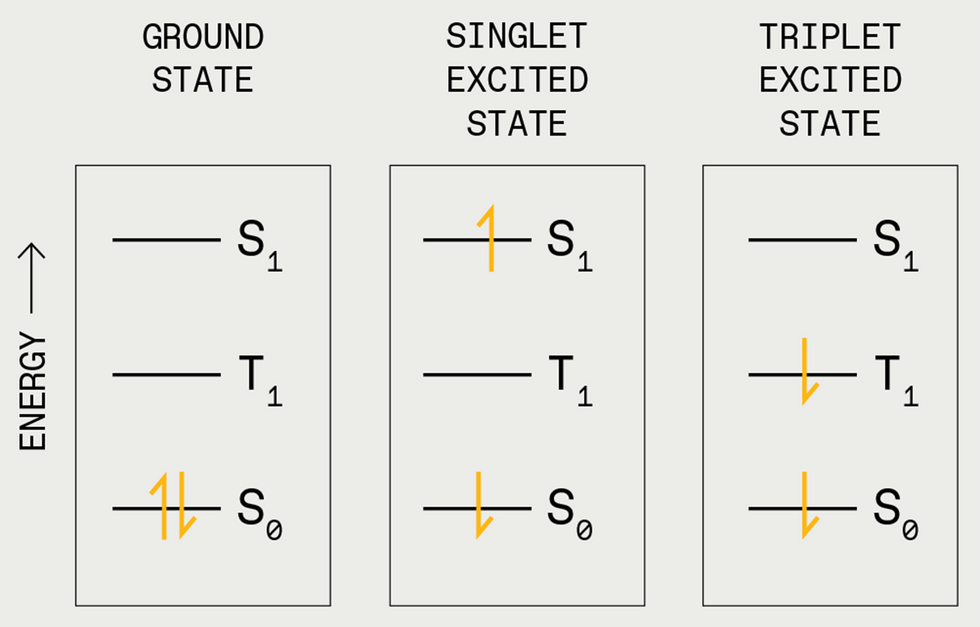 Electrons occupy the bottom state [S0] in pairs with reverse “spins” [up and down arrows, left]. A photon can kick one of many electrons into the singlet excited state [S1, center]. Normally, the electron will shortly fall again to the bottom state and emit a photon. However generally the electron’s spin can flip, and it will get caught at a decrease power stage, the triplet excited state [T1, right].
Electrons occupy the bottom state [S0] in pairs with reverse “spins” [up and down arrows, left]. A photon can kick one of many electrons into the singlet excited state [S1, center]. Normally, the electron will shortly fall again to the bottom state and emit a photon. However generally the electron’s spin can flip, and it will get caught at a decrease power stage, the triplet excited state [T1, right].
If a photon whose power matches the bandgap of the semiconductor hits a photo voltaic cell, this course of proceeds easily: The incident photon generates an excited electron that’s successfully captured to generate electrical energy. If a photon has an power higher than the bandgap of the fabric—as is the case for all seen mild for many photovoltaic supplies in use—the incident photon generates an electron greater in power. This excited electron then quickly relaxes to an power equal to the bandgap, and all the surplus power is misplaced as warmth, a waste for the photo voltaic cell. Even worse, photons with much less power than the bandgap can’t do something productive in any respect, and easily go by the semiconductor unabsorbed.
This presents a troublesome trade-off for the solar-cell designer: Wider bandgaps will lose much less as warmth however soak up fewer photons, whereas narrower bandgaps will soak up extra of the obtainable photons however lose extra as warmth. Silicon, the ever-present light-absorbing photovoltaic materials that makes up greater than 90 p.c of as we speak’s photovoltaic market, sits within the candy spot of this trade-off. Nonetheless, even the most effective experimental silicon photo voltaic cells depart virtually three-quarters of the obtainable daylight energy unharvested.
This irritating state of affairs has lengthy impressed scientists and engineers, together with us, to seek for a greater method.
One promising concept is to make use of a number of absorber supplies to create a
stack of photo voltaic cells during which every semiconductor is paired with a selected portion of the photo voltaic spectrum. Designing these cells might be tough. As an example, in a single configuration, every subcell should output the identical quantity of present; in any other case, effectivity can be restricted to that of the worst-performing subcell. At present, probably the most environment friendly machine made utilizing three mild absorbers underneath customary illumination—that’s, with out utilizing lenses or different concentrators—has an effectivity of 39.5 p.c.
However we expect that altering the colour of sunshine can additional increase efficiencies: As an alternative of attempting to match the cell to the incoming mild, we are able to match the sunshine to the cell.
Which means we convert the photons beneath the photo voltaic cells’ bandgap to harvestable, higher-energy photons. In the previous couple of years in our lab at Stanford and in collaboration with different scientists all over the world, now we have efficiently upconverted low-energy infrared photons—which regularly can’t be utilized by as we speak’s photo voltaic cells—into productive yellow photons that may. And we translated this chemistry, initially developed in a beaker, right into a thin-film materials.
We’re learning tips on how to enhance these features by controlling how power strikes inside our supplies, how the singlet and triplet states work together, and the way the sunshine is emitted from the skinny movie to a photo voltaic array. Scientists all over the world, together with us, are working to develop supplies that may allow more-efficient upconversion programs that harvest even additional into the infrared. This expertise isn’t getting used commercially but, however we consider it can get there.
Utilizing Colour Adjustments to Hit a Goal
Enhancing the effectivity of photo voltaic cells is way from the one thrilling use for altering the colour of sunshine by upconversion. This expertise can be used to focus on mild to a exact location, fixing an issue frequent to biology, chemistry, and additive manufacturing.
Stopping undesirable absorption or scattering of sunshine is necessary in functions as various as activating a drug at a tumor website, lighting up a neuron to review mind perform, and—maybe surprisingly—exactly constructing constructions by additive manufacturing. The best way we remedy this problem is analogous in every case, however additive manufacturing (3D printing) is especially promising and maybe the best to clarify.
If one have been to think about the easiest way to print a form in three dimensions, with out utilizing as we speak’s expertise, it’s simple to image curing particular person factors at their
x, y, and z coordinates inside a vat of resin. But curing a single goal level with out curing the area round it’s troublesome. Shining a laser beam into the resin, for example, cures it alongside the complete laser path.

However we are able to get to this stage of precision by altering the colour of sunshine. Right here’s how that works.
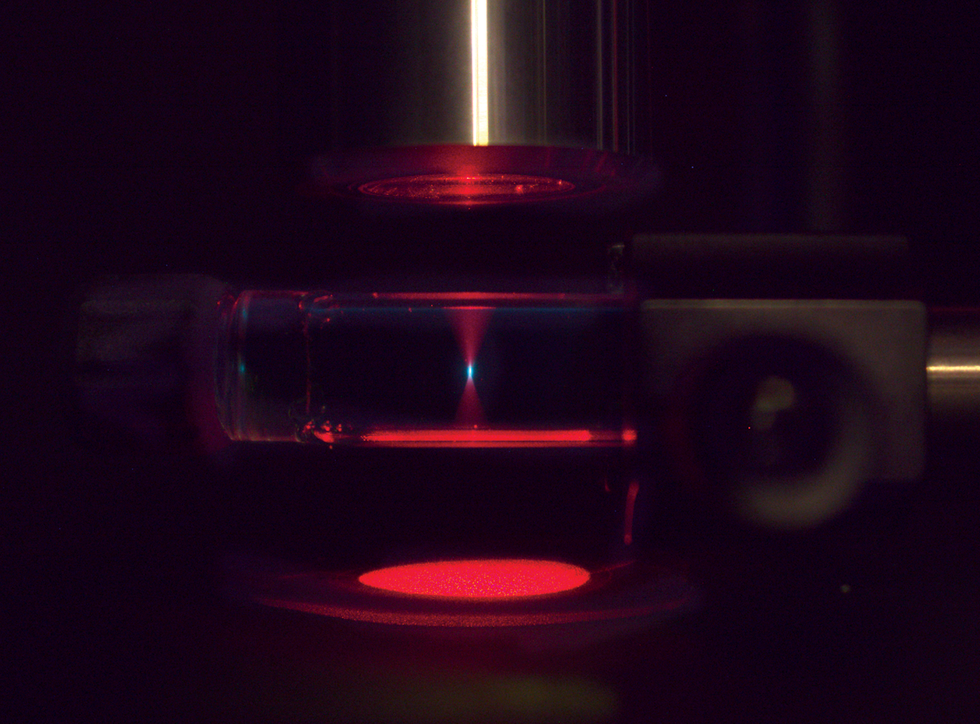 To print this 3D boat [top], supplies that may change the colour of sunshine have been dispersed in a pool of resin. Focusing a purple laser at some extent within the pool [above] triggered upconversion, making a dot of blue mild that cured the resin at that spot. Transferring the laser in three dimensions constructed the boat dot by dot.STANFORD UNIVERSITY/HARVARD UNIVERSITY/NATURE
To print this 3D boat [top], supplies that may change the colour of sunshine have been dispersed in a pool of resin. Focusing a purple laser at some extent within the pool [above] triggered upconversion, making a dot of blue mild that cured the resin at that spot. Transferring the laser in three dimensions constructed the boat dot by dot.STANFORD UNIVERSITY/HARVARD UNIVERSITY/NATURE
Inside
our upconversion 3D printer we use a resin containing dispersed nanoparticles with sensitizers and annihilators. In 3D printing, blue or UV photons are usually used to drive the curing of resin, however we don’t begin with blue mild. As an alternative, we shine a purple laser beam towards our goal.
Then we benefit from the truth that upconversion occurs solely at sure intensities of sunshine: We use a lens to focus our purple beam on a selected level within the resin pool, growing its depth at that spot. Upconversion creates a small dot of blue mild at the focus of the purple mild, curing the resin on the dot. By transferring the focus round, we are able to create arbitrary shapes deep in our resin pool. What’s thrilling is that this complete course of might be run with a laser no extra highly effective than a typical laser pointer. We now have already made a lot of pattern objects, together with a toy boat, a gear, and a few Stanford and Harvard College logos.
Transferring ahead, we’re significantly enthusiastic about utilizing this method to quickly print many objects on the nanoscale in parallel—one thing that has been troublesome to do, since focusing too many high-powered lasers into one pool of resin merely breaks the resin down earlier than it may be reworked right into a strong plastic. The low-power lasers used for upconversion don’t do that.
Nonetheless additional promising developments stay past these functions: Upconversion might enable for near-infrared beams, which penetrate deep into residing tissue, to generate high-energy photons helpful for deep-tissue imaging, optogenetics, and native chemical reactions.
Lastly, we’re additionally exploring functions like passive night-vision programs and sturdy anticounterfeiting schemes. Every of those functions would require a skinny coating of upconversion supplies on a floor, in the identical method we’re utilizing our expertise with photo voltaic cells. Think about buying a pair of glasses with an added upconversion coating that means that you can see infrared photons, enhancing night time imaginative and prescient with out the cumbersome energy supply required in as we speak’s night-vision goggles. Or, in the event you embedded upconversion nanoparticles in forex or ID playing cards, distinguishing actual from counterfeit can be as simple as shining a purple laser pointer on the floor and seeing the sunshine flip blue.
Though every software requires several types of supplies, getting high-energy photons to the fitting place by upconversion can be utilized to kick-start each. We’re solely starting to scratch the floor of what this expertise can do.
From Your Website Articles
Associated Articles Across the Internet