In an effort to improve the accessibility of hydrogen-powered automobiles and set up hydrogen as a viable power supply, it is crucial to scale back the price of hydrogen manufacturing, thereby attaining financial feasibility. To realize this purpose, maximizing the effectivity of electrolysis-hydrogen evolution, the method accountable for producing hydrogen from water, is essential.
Just lately, a workforce of researchers comprising Professor In Su Lee, Analysis Professor Soumen Dutta, and Byeong Su Gu from the Division of Chemistry at Pohang College of Science and Know-how (POSTECH) achieved a major enchancment in manufacturing effectivity of hydrogen, a inexperienced power supply, via the event of a platinum nanocatalyst. They completed this feat by depositing two totally different metals in a stepwise method.
The findings of their analysis have been printed in Angewandte Chemie.
Depositing distinct supplies selectively on particular places of a catalyst floor, whose measurement is within the nanometer vary, poses substantial challenges. Unintended depositions might block the catalyst’s lively websites or intervene with one another’s capabilities. This predicament has prevented the simultaneous deposition of nickel and palladium onto a single materials. Nickel is accountable for activating water splitting whereas palladium facilitates the conversion of hydrogen ions into hydrogen molecules.
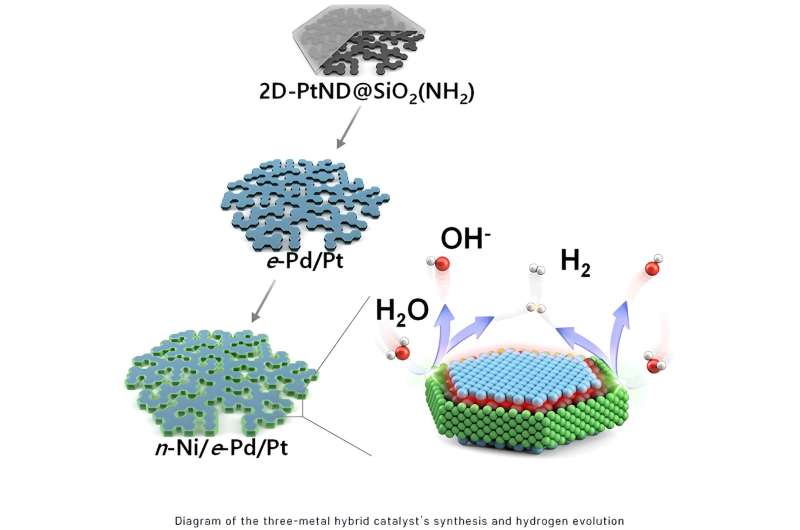
The analysis workforce developed a novel nano reactor to finely management the situation of metals deposited onto a 2D flat nanocrystal. Moreover, they devised a nano-scaled advantageous deposition course of, enabling the protection of various sides of the 2D platinum nanocrystal with totally different supplies.
This new method led to the event of “platinum-nickel-palladium” three-metal hybrid catalyst materials achieved via consecutive depositions that selectively cowl the flat floor and the sting of the 2D platinum nanocrystal with palladium and nickel nano skinny movies respectively.
The hybrid catalyst featured distinct nickel/platinum and palladium/platinum interfaces positioned to facilitate the water splitting and hydrogen molecule era processes respectively. Consequently, the collaborative incidence of those two totally different processes considerably boosted the effectiveness of electrolysis-hydrogen evolution.
The analysis outcomes revealed that the three-metal hybrid nanocatalyst exhibited 7.9-fold enhance in catalytic exercise in comparison with the traditional platinum-carbon catalyst. Furthermore, the novel catalyst demonstrated vital stability, sustaining its excessive catalytic exercise even after a protracted 50-hour response time. This resolved the difficulty of purposeful interferences or collisions between heterointerfaces.
Professor In Su Lee, who led the analysis, says, “We’ve efficiently developed harmonious heterointerfaces fashioned on a hybrid materials, overcoming the challenges of the method.” He additional added, “I hope the analysis findings will discover widespread software within the improvement of catalytic supplies optimized for hydrogen reactions.”
Extra info:
Byeong Su Gu et al, Harmonious Heterointerfaces Shaped on 2D‐Pt Nanodendrites by Aspect‐Respective Stepwise Steel Deposition for Enhanced Hydrogen Evolution Response, Angewandte Chemie Worldwide Version (2023). DOI: 10.1002/anie.202307816
Supplied by
Pohang College of Science and Know-how
Quotation:
Analysis workforce enhances hydrogen evolution catalyst via stepwise deposition (2023, August 22)
retrieved 26 August 2023
from https://phys.org/information/2023-08-team-hydrogen-evolution-catalyst-stepwise.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.

