The diminishing typical power assets based mostly on fossil fuels and their associated environmental penalties have drawn consideration around the globe towards the event of renewable power assets. These renewable power assets could not fulfill your complete power calls for of world’s mass inhabitants; nevertheless, they restrict the consequences of greenhouse gases in addition to air air pollution brought on by the fossil gas burning. Amongst various assets, hydrogen is taken into account to be the cleanest power provider.
Nevertheless, hydrogen doesn’t exist in its pure state in nature, like oxygen, and needs to be produced from hydrogen-containing assets similar to pure fuel (methane), coal, biomass and water by reforming, thermal decomposition or electrolysis. However manufacturing of hydrogen from pure fuel, coal and biomass results in the emission of the greenhouse fuel carbon dioxide (CO2).
We all know that water (H2O) is fabricated from hydrogen and oxygen atoms; therefore, sea water could possibly be a limitless supply of hydrogen. Due to this fact, hydrogen is envisaged as a potential alternative for fossil fuels. Manufacturing through energy from renewable power (utilizing wind energy, solar energy, hydropower, wave energy or comparable) is termed as “inexperienced hydrogen.” On this state of affairs, splitting water into hydrogen and oxygen utilizing renewable electrical energy in an electrolyzer on the floor of a strong electrocatalyst is one proposed approach.
Want for a strong electrocatalyst
Regardless of developments within the area, the method of water spitting to supply inexpensive inexperienced hydrogen nonetheless stays sluggish attributable to limitations associated to environment friendly electrocatalysts. In principle, water splits at 1.23 V. Nevertheless, in sensible phrases, this worth is bigger than 1.5 V (that means wastage of extra power). This minimal power is theoretically required to interrupt the water molecule. Costly noble- and precious-metal-based electocatalysts, as an illustration, Pt, Pd, Au, Rh, Ir, and many others., are used within the electrolyzer for this course of.
The primary issues the trade and consultants face are the oxidation of water to supply O2 and the steadiness of the catalyst in harsh industrial alkaline situations. Within the first drawback, the half-cell response is an uphill response the place 4 electrons are concerned and the place a lot of the power is required other than the power loss related to the resistivity of various elements (electrolyte, connections, catalyst, and many others.) of the electrolyzer. Within the second drawback, the costly catalysts usually lose their exercise attributable to floor degradation. In these situations, an inexpensive and inexpensive but extremely lively and steady electrocatalyst is required for such water splitting response.
Current growth
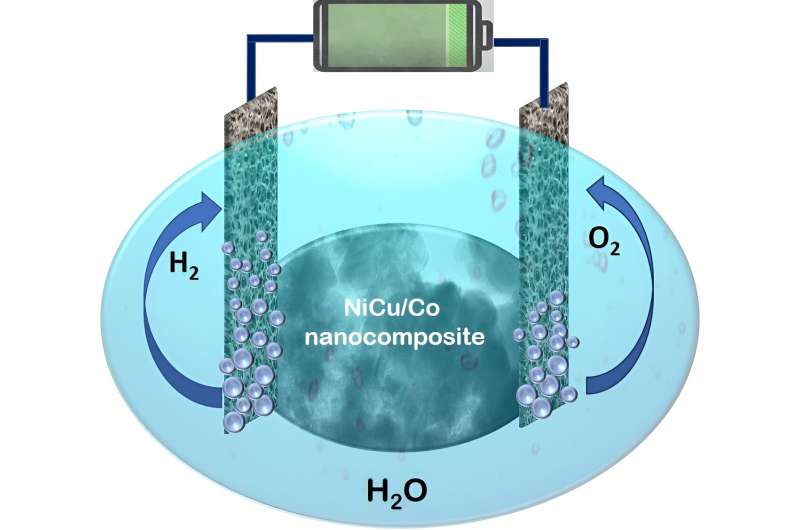
In a latest examine, our group, led by Sasanka Deka, designed and developed a brand new nanocomposite-based, extremely environment friendly, but nonetheless cost-effective electrocatalyst for total water splitting. A nanocomposite is a homogeneous combination of two or extra supplies current within the nanometer vary. The current nanocomposite is a nanoarchitecture based mostly on NiCu dealloyed nanoparticles on hierarchical Co nanosheets. Our findings are printed within the journal ACS Catalysis.
The supplies used are cheaper than the dear metals and the synthesis process is extremely handy. This new catalyst was utilized in an electrolyzer in potassium hydroxide (KOH) electrolyte for the splitting of water. Curiously, the system reveals the splitting of water and manufacturing of hydrogen fuel utilizing the NiCu/Co electrocatalyst at 1.46 V cell voltage. Thus, the electrocatalyst is able to splitting of water by utilizing solely a family 1.5-volt battery.
Different key factors in regards to the NiCu/Co electrocatalyst are the inexperienced hydrogen manufacturing takes place with industrially necessary excessive present density, excessive stability (6,000 cycles) and sturdiness (60 h) of the catalyst. It additionally works on industrial electrolyte situation of 30 wt.% KOH electrolyte and the cell voltage supplied is way decrease than that of a business IrO2||Pt/C catalyst.
Detailed experimental and computational research have been carried out to grasp the rationale behind this effectivity. The corroborated outcomes assist our preliminary speculation of selective leaching of supplies to make a extra porous construction, and the usage of totally different metallic facilities and shapes of supplies for hydrogen and oxygen evolution.
In abstract, we have now developed a easy however superior and cost-effective methodology to design a nanocomposite-based, bifunctional electrocatalyst of dealloyed NiCu on Co nanosheets that may break up water at 1.46 V with nice stability. We hope that our product could possibly be helpful for scale-up synthesis and business use in electrolyzers for inexperienced hydrogen manufacturing.
This story is a part of Science X Dialog, the place researchers can report findings from their printed analysis articles. Go to this web page for details about ScienceX Dialog and learn how to take part.
Extra data:
Ankur Kumar et al, Designing Nanoarchitecture of NiCu Dealloyed Nanoparticles on Hierarchical Co Nanosheets for Alkaline Total Water Splitting at Low Cell Voltage, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c02096
Dr. Sasanka Deka is Professor of Chemistry, College of Delhi. He obtained his Ph.D. diploma from Nationwide Chemical Laboratory (NCL-Pune). He did his postdoctoral analysis from Nationwide Nanotechnology Laboratory, CNR-INFM, Lecce, Italy and Italian Institute of Expertise (IIT), Genova, Italy. He has been awarded the TMS Basis 2008 SHRI RAM ARORA AWARD, by the Minerals, Metals & Supplies Society (TMS), Warrendale, USA; DAE-BRNS Younger scientist analysis award 2011, RSC finest oral speak–2015, Institute of Physics (IOP), UK finest cited paper-India 2019 and RSC finest cited paper in 2020. Dr. Deka has printed greater than 75 analysis papers in several worldwide excessive impression journals, holds three patents, and in addition wrote two books and three e book chapters printed by a world writer. He has efficiently dealt with a number of extramural nationwide and worldwide analysis initiatives. His present analysis curiosity offers with artificial nanochemistry, and novel nanomaterials for power analysis.
Quotation:
Nanocomposite-based electrocatalyst for alkaline total water splitting at low cell voltage for hydrogen manufacturing (2023, August 30)
retrieved 2 September 2023
from https://phys.org/information/2023-08-nanocomposite-based-electrocatalyst-alkaline-cell-voltage.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.

