This research was collaboratively carried out by Dr. Shu-Hong Yu, Dr. Lengthy-Ping Wen, and Dr. Kun Qu from the College of Science and Know-how of China, with Professor Yang Lu from the Hefei College of Know-how.
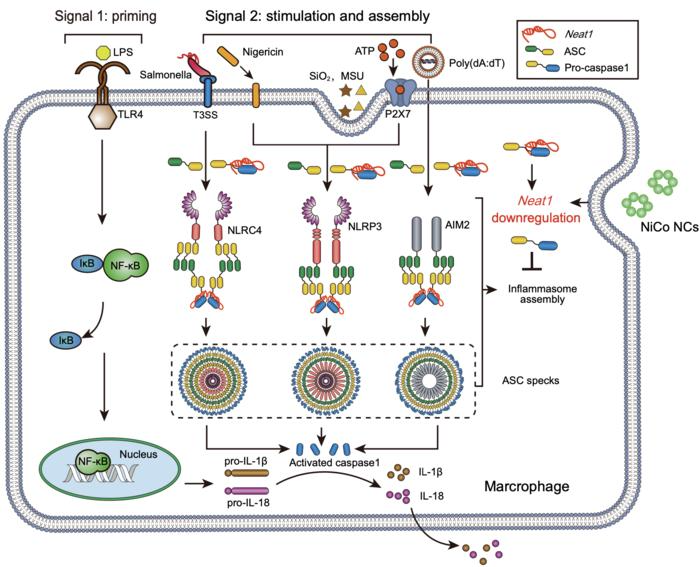
A number of stimuli set off the activation of three inflammasomes: NLRP3, NLRC4, and AIM2. NiCo NCs suppressed the inflammasome meeting course of by downregulating the lengthy non-coding RNA Neat1, thereby inhibiting the activation of three inflammasomes. Picture Credit score: ©Science China Press
An excessive amount of activation of inflammasomes has been linked to a number of ailments, reminiscent of atherosclerosis, Alzheimer’s illness, gout, and sort 2 diabetes.
Supplied the pivotal position of macrophages in each nanoparticle phagocytosis and inflammasome activation, the breakthrough of anti-inflammatory nanoparticles, notably concentrating on macrophages, may extra effectively modulate inflammatory response whereas decreasing off-target results in different cell varieties.
However most presently reported nanomaterials have been found to encourage as a substitute of hinder inflammasome activation.
The research confirmed that nickel-cobalt alloy nanocrystals show excellent efficacy in suppressing the activation of three inflammasomes in predominant macrophages, specifically NLRP3, AIM2, and NLRC4.
Later, the scientists employed two illness fashions, colitis and acute peritonitis, to evaluate the impact of nickel-cobalt alloy nanocrystals on treating inflammasome overactivation.
The outcomes confirmed how nickel-cobalt alloy nanocrystals effectively ameliorated illness signs in mice within the colitis mannequin, reminiscent of restoring colon size, mitigating weight reduction, and mitigating injury to the intestinal mucosal epithelium. Furthermore, within the acute peritonitis mannequin, such nanocrystals significantly attenuated neutrophil chemotaxis contained in the peritoneal cavity of mice.
To confirm if nickel-cobalt alloy nanocrystals want mobile internalization to use their anti-inflammatory results, the authors carried out experiments with the assistance of an also used endocytosis inhibitor named cytochalasin D.
Remedy carried out with cytochalasin D significantly decreased the internalization of nickel-cobalt alloy nanocrystals by macrophages. Furthermore, hindering the internalization of the nanocrystals by macrophages has resulted in a lower of their anti-inflammatory results. This reveals that the anti-inflammatory motion of nickel-cobalt alloy nanocrystals is dependent upon their mobile uptake.
To investigate if the anti-inflammatory impacts of nickel-cobalt alloy nanocrystals are assigned to their elemental composition or geometric morphology, the authors synthesized cobalt nanoparticles and nickel nanoparticles beneath equivalent situations as controls. This displayed various morphologies compared to the nickel-cobalt alloy nanocrystals.
However each cobalt nanoparticles and nickel nanoparticles additionally significantly restrained inflammasome activation.
Therefore, the authors assigned the inhibitory impact of nickel-cobalt alloy nanocrystals to the fundamental composition as a substitute of their geometric form. Such findings denote that nanomaterials consisting of cobalt and nickel would possibly present alternatives for growing nano-drugs with anti-inflammatory properties.
Disclosing the organic mechanisms underlying the motion of nanomaterials is taken into account important for his or her doable medical functions. However clarifying the organic mechanism by which such broad-spectrum anti-inflammatory nanocrystals curb inflammasome activation poses appreciable difficulties utilizing conventional organic experimental approaches.
To meet this, scientists carried out RNA sequencing and the assay for transposase-accessible chromatin with sequencing (ATAC-Seq). This resulted within the identification of an earlier reported non-coding RNA, Neat1, thought-about to be concerned in inflammasome meeting.
After therapy with the nickel-cobalt alloy nanocrystals, the expression of Neat1 was significantly decreased. Earlier research have illustrated that downregulating Neat1 expression alone considerably prevents the activation of NLRC4, NLRP3, and AIM2 inflammasomes.
ATAC-Seq outcomes disclosed a substantial lower within the chromatin accessibility of the gene physique and promoter areas of Neat1 within the nickel-cobalt alloy nanocrystal-treated group. This denotes that the inhibition of inflammasome activation by the nickel-cobalt alloy nanocrystals has been obtained by suppressing Neat1 transcription as a substitute of fostering its degradation.
Journal Reference
Lin, J., et al. (2023) Nickle-cobalt alloy nanocrystals inhibit activation of inflammasomes. Nationwide Science Evaluate. doi.org/10.1093/nsr/nwad179.
