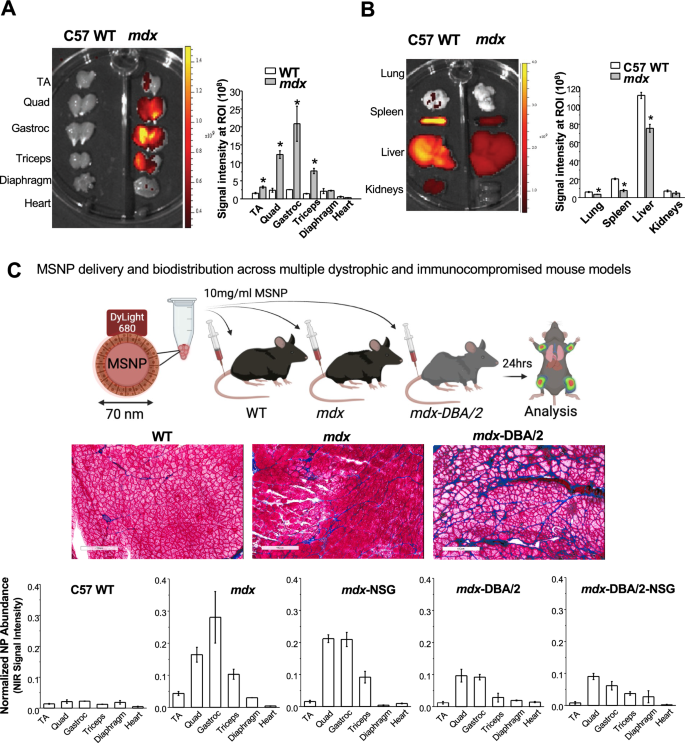
MSNPs enhance biodistribution to dystrophic muscle groups in comparison with regular muscle groups in a number of DMD murine fashions
We chosen MSNPs with a major diameter of 70 nm as our group has beforehand optimized this dimension for systemic penetration into stable tumors [25, 26, 34]. To trace biodistribution of MSNPs on the tissue and mobile stage in dystrophic muscle groups, purified MSNPs had been floor functionalized with amino teams which enabled conjugation of amine reactive near-infrared (NIR) dye. Additional coating MSNPs with uniform lipid bilayers endowed the nanocarriers with enhanced colloidal stability and minimized non-specific adsorption of serum proteins by nanoparticles in blood and delay their circulation time [33].
To guage MSNP biodistribution, we carried out a comparative evaluation on age matched wholesome C57BL/6 (WT) versus mdx mice. Mice acquired a single IV injection of 10 mg/mL NIR dye labeled MSNPs at 50 mg silica dose per kg by way of the tail vein and had been sacrificed 24 h publish injection. Skeletal and cardiac muscle groups had been dissected and instantly imaged ex vivo utilizing the IVIS imaging system (Fig. 1A). Opposite to the distribution in wildtype animals, we noticed preferential and considerably increased MSNP retention in all limb skeletal muscle groups of dystrophic mice. This profile was utterly modified in wildtype muscle groups wherein low NIR sign was detected. MSNPs confirmed robust NIR fluorescence depth in higher and decrease limb skeletal muscle groups together with gastrocnemius, quadriceps, and triceps exhibiting 6–tenfold better MSNP abundance in comparison with wildtype mice, p < 0.05. Quantitative evaluation of sign depth additionally confirmed that MSNP distribution is muscle sort dependent. Gastrocnemius (gastroc) and quadriceps produced 3–5 folder increased sign than tibialis anterior (TA) from the identical mice. The NIR indicators within the diaphragm and coronary heart had been weakest suggesting MSNP retention in cardiac and skeletal muscle groups happen by way of separate mechanisms.
IV injection of MSNPs biodistribute in dystrophic muscle groups of mdx mice however not regular muscle tissues in extensive sort mice. A and B Ex vivo NIR fluorescence imaging of muscle groups and organs at 24 h after animals acquired a single IV injection of NIR-labeled lipid coated 70 nm MSNPs (50 mg/kg). NIR fluorescence depth at ROIs was used to quantify the nanoparticle content material in numerous muscle groups and organs. Knowledge characterize imply ± SEM (n = 3). *P < 0.05, 2-tailed Pupil’s t take a look at. C-middle Masson’s Trichome staining of TA and Gastroc in 3-month previous male C57BL/6, mdx mice and mdx-D2 mice to exhibit the extent of fibrosis with collagen deposit (blue colour) in muscle groups of the totally different mouse fashions. Bars represents 100 μm. C-bottom Relative biodistribution of NPs at muscle websites in C57BL/6 WT and totally different DMD mouse fashions at 24 h after acquired IV injection of NIR labeled NPs. The fluorescence intensities of muscle groups had been normalized to the liver for every animal. Knowledge characterize imply ± SEM (n = 3)
Along with muscle groups, we additionally harvested the main organs of the reticuloendothelial system (RES; (liver and spleen)), kidneys, and lungs (Fig. 1B). Whereas IV injection of MSNPs led to important RES organ uptake in regular mice, we discovered a major discount in RES uptake in mdx mice. Most notably, MSNP sign was decreased within the spleen and liver by threefold and 1.5-fold in mdx mice, respectively. That is the primary demonstration of a nanoparticle to selectively goal dystrophic skeletal muscle groups as in comparison with off-target RES organs, presumably because of the aggressive MSNP retention inside dystrophic mdx muscle groups (Fig. 1B).
MSNP bio-distribution is decreased in fibrotic muscle websites however isn’t affected in immunocompromised mice
The elevated MSNP biodistribution in mdx skeletal muscle prompted us to ask if the preferential MSNP uptake and retention relies upon the illness state of skeletal muscle. We hypothesized that elevated illness severity would lead to better MSNP supply to the muscle groups. Accordingly, we carried out biodistribution research in mdx-DBA/2 mice, a extra fibrotic and severely diseased mannequin which higher recapitulates a number of of the human traits of DMD myopathology [35, 36]. Just like earlier, mdx-DBA/2 mice had been injected with NIR Dye-conjugated MSNPs and after 24 h skeletal muscle and main organs had been dissected and imaged with IVIS (Fig. 1C). Surprisingly, nonetheless, we discovered extra plentiful MSNP retention in mdx fashions in comparison with the extra extreme mdx-DBA/2 fashions.
We subsequent used mdx and mdx-DBA/2 mice that had been crossed to immunocompromised (Nod-Scid-Gamma (NSG)) mice, poor in T-cell, B-cell, and NK-cells, to check if MSNP biodistribution was regulated by the immune system [37]. We discovered no important variations in MSNP biodistribution in mdx-NSG in contrast to mdx mice, and no important distinction between mdx-DBA/2-NSG in contrast mdx-DBA/2 mice (Fig. 1C). Slightly, variations had been primarily noticed between the mouse strains and never because of the absence of an adaptive immune system. Plentiful MSNP biodistribution to skeletal muscle was confirmed in cross sections of each mouse fashions utilizing NIR immunofluorescence, in addition to laminin and a clean Cy3 channel which accounted for background (Fig. 1C).
MSNP biodistribution didn’t correlate with numbers of skeletal muscle capillaries and blood vessels throughout muscle teams nor dystrophic mouse fashions
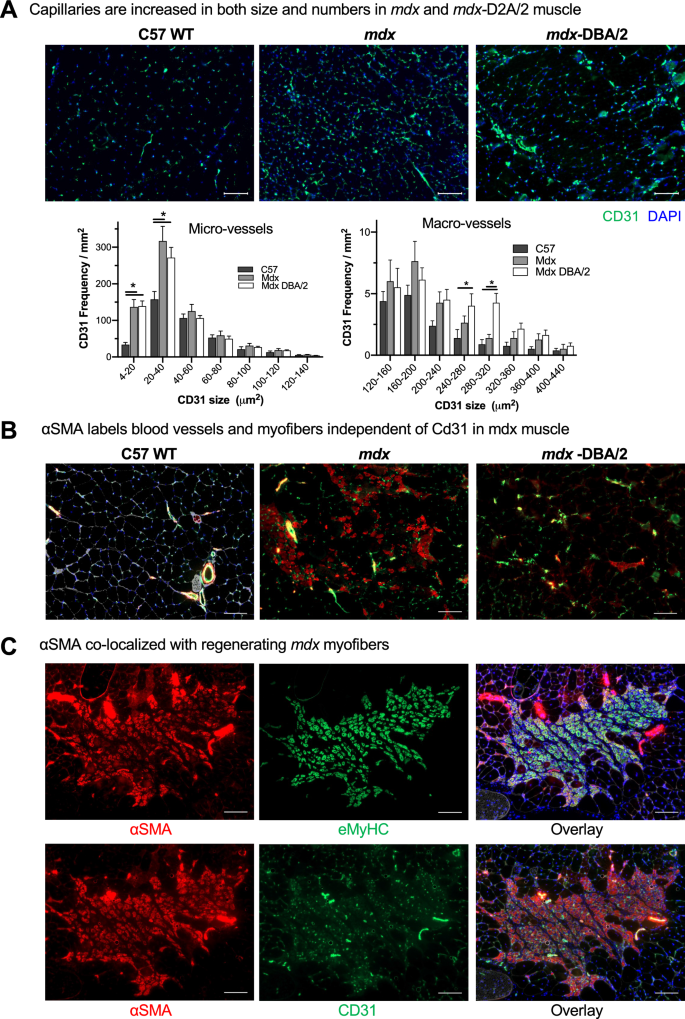
As nanoparticles traditionally don’t biodistribute effectively to skeletal muscle [38, 39], we got down to establish the mechanism for MSNP accumulation in skeletal muscle groups and decide if we may additional enhance muscle homing. We first quantified blood vessel numbers (marked by Cd31) and cross-sectional space from quadriceps of C57BL/6 WT, mdx, and mdx-DBA/2 mice. We discovered an elevated variety of blood vessels in each the mdx and mdx-DBA/2 mice in comparison with age-matched WT muscle, p < 0.05. Particularly, CD31 optimistic micro-vessels (between 4 and 40 μm2) elevated between dystrophic and wildtype mice (Fig. 2A). Nevertheless, the variations in micro-vessel density between mdx and mdx-DBA/2 mice weren’t statistically totally different, and thus alone couldn’t account for biodistribution modifications between these mouse fashions. In reality, mdx-DBA/2 mice had a better variety of massive vessels (between 200 and 300 μm2), which didn’t correlate with elevated MSNP biodistribution to the much less dystrophic mdx mice.
Dystrophic muscle groups differ of their degenerative/regenerative states which correlates to MSNP biodistribution. A. Consultant microscopy photos of CD31 immunofluorescence (IF) staining of tibialis anterior (TA) and gastrocnemius (Gastroc) in 3-months male C57BL/6 mice, mdx and mdx-DBA/2 mice. Coloration code: inexperienced, Cd31; blue, nuclear. Scale bar represents 50 μm. Histogram evaluation of Cd31 + blood vessel density and dimension exhibits quantification from greater than 8 randomly chosen areas. Knowledge characterize imply ± SEM. *P < 0.05, 1-way ANOVA adopted by Tukey’s take a look at. B. αSMA labels blood vessels and myofibers impartial of Cd31 in mdx muscle. Bars represents 100 μm. C. αSMA co-localizes with regenerating mdx myofibers. Scale Bar represents 50 μm
In parallel, we investigated why particular skeletal muscle groups equivalent to quadriceps and gastrocnemius had elevated MSNP numbers in comparison with tibialis anterior. We discovered no important variations or developments in endothelial cell density, pericyte protection, or trichrome staining between gastrocnemius and tibialis anterior in mdx or mdx-DBA/2 mice that accounted for the 6–tenfold MSNP enhance in gastrocnemius biodistribution (Further file 1: Fig. S1). We subsequent hypothesized that muscle-specific purposeful use and blood perfusion [40] could differentially regulate MSNP biodistribution, and thus carried out a extra detailed vasculature evaluation utilizing endothelial (CD31) and {smooth} muscle (α-SMA) colocalization to tell apart massive blood vessels from capillaries. In wildtype mice, CD31 and α-SMA constantly colocalized demarcating massive blood vessels as anticipated (Fig. 2B). Apparently, within the mdx mice we discovered a hanging enhance in α-SMA non-colocalized CD31. In mdx mice, the α-SMA + staining was particular to centrally nucleated myofibers, which is a trademark of muscle regeneration myofibers (Fig. 2B, C). Nevertheless, in mdx-DBA/2 muscle α-SMA + myofibers had been significantly decreased (Fig. 2B). Earlier stories have proven α-SMA is transiently expressed by regenerating myofibers [41], thus we stained muscle serial sections for the regenerative marker embryonic myosin (eMyHC). Certainly in mdx, we discovered dozens of α-SMA + myofibers co-localized with eMyHC + (Fig. 2C). Thus, whereas we initially hypothesized modifications in blood vessel density would enhance MSNP biodistribution, we as a substitute recognized that modifications in muscle regeneration instantly corresponded with elevated MSNP biodistribution throughout a number of mouse fashions.
MSNPs biodistribute to websites of regenerating skeletal muscle
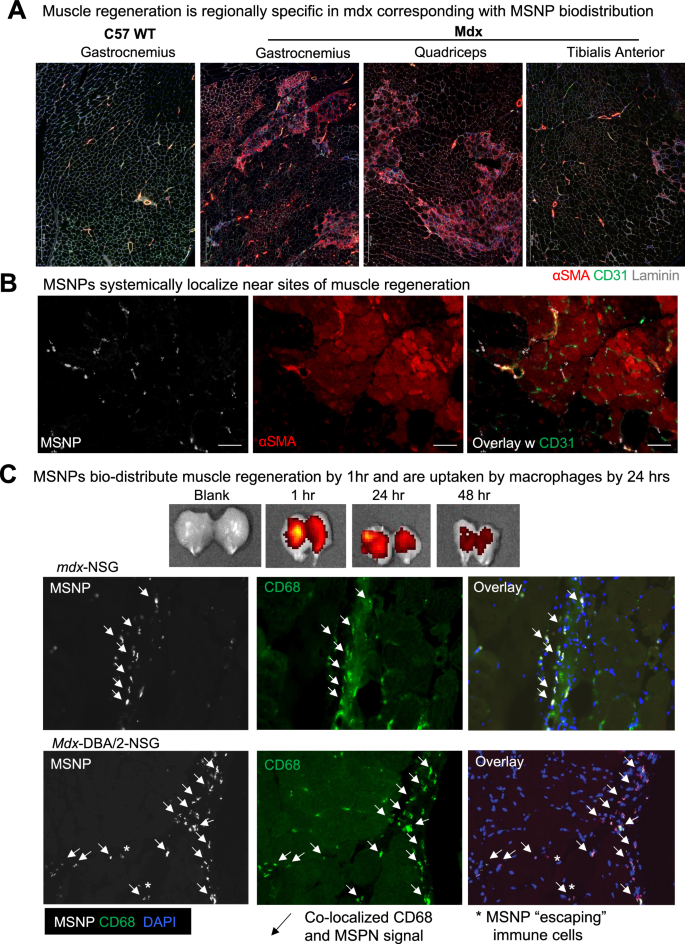
We subsequent evaluated websites of muscle regeneration throughout the muscle teams: gastrocnemius, quadriceps, tibialis anterior in mdx and wildtype mice. Simply as NIR-labeled MSNP had enhanced biodistributed to gastrocnemius and quadriceps, we discovered the next frequency of α-SMA + myofibers inside these muscle groups and fewer α-SMA + myofibers in mdx TA muscle groups. Now we have included zoomed out tiled photos that present this substantial enhance in α-SMA + myofibers (Fig. 3A-B). Not like mdx, C57 wildtype gastrocnemius confirmed no α-SMA + myofibers which additionally correlated with the little-to-no uptake of MSNPs.
To verify elevated MSNP biodistribution at websites containing regenerating myofibers, we once more delivered NIR-labeled MSNPs to mdx mice and after 24 h evaluated muscle cross sections for co-localization of nanoparticles with CD31 or α-SMA + or eMYHC + Laminin + myofibers. As anticipated, we discovered elevated MSNPs at websites of α-SMA + myofibers (Fig. 3A). That is essential given the mobile composition of dystrophic skeletal muscle is heterogeneous containing area of regeneration, necrosis, and comparatively regular myofibers, and we discovered elevated NIR-labeled MSNPs aggregated close to websites of regenerating myofibers. We due to this fact concluded that MSNPs predominately bio-distribute inside tissues to particular websites of regenerating myofibers which may very well be because of the MSNPs getting into the muscle after damage, and regeneration following muscle damage.
MSNPs biodistribute to regenerating myofibers in dystrophic muscle. A–B. Staining of muscle regeneration. Tiled photos of gastrocnemius, quadriceps, and tibialis anterior muscle groups exhibits various ranges of regeneration throughout and corresponds with MSNP biodistribution. Scale bars represents 500 μm. B–C. Time course of MSNP biodistribution to skeletal muscle. MSNPs packaged with NIR labeled protein bio-distribute to skeletal muscle by 1 h and are cleared by 48 h. IVIS of skeletal muscle tissue at 1, 24, and 48 h publish MSNP systemic supply (50 mg/kg). Staining exhibits MSNPs (white), regenerating myofibers (a-SMA, crimson), capillaries (Cd31, inexperienced), and nuclei (DAPI, blue). Scale bars characterize 20 μm. Coloration code: Purple, vascular smooth-muscle cells stained with anti-alpha {smooth} muscle actin (α-SMA) antibody, nuclear stained with DAPI; white, NIR MSNPs. Co-staining of Cd68 + macrophages (inexperienced) with MSNPs (white) exhibits better than 90% co-localization at 24 h publish systemic injection
MSNPs are detected in regenerating muscle by 1 h and quickly cleared by macrophages
To find out dynamics of MSNPs getting into and clearance from the muscle, we subsequent carried out a time course evaluation of MSNP biodistribution. For these research, a protein customary cytochrome-c was labeled with NIR dye and packaged in MSNPs for evaluation. By IVIS imaging, we demonstrated MSNPs might be robustly detected inside skeletal muscle by 1 h and the sign is considerably decreased inside 48 h of systemic injection (Fig. 3C). Skeletal muscle groups had been dissected and cross sections imaged at 1, 4, 24, and 48 h, which discovered MSNPs inside the muscle capillaries at 1 h and current the vasculature into the muscle stroma by 4 h. By 24 h, better than 90% of MSNPs had been uptaken by CD68 + macrophages in all mouse fashions together with immunocompromised mdx-NSG and mdx-DBA/2-NSG mice, suggesting that macrophages are taking part in a task in clearance or transport of MSNPs to regenerative websites within the muscle (Fig. 3C and S3). These outcomes point out that systemic MSNP injection would allow speedy imaging of broken muscle and could be transiently retained for brief intervals of 1–2 days.
Experimentally induced damage validates MSNPs bio-distribute to regenerating skeletal muscle
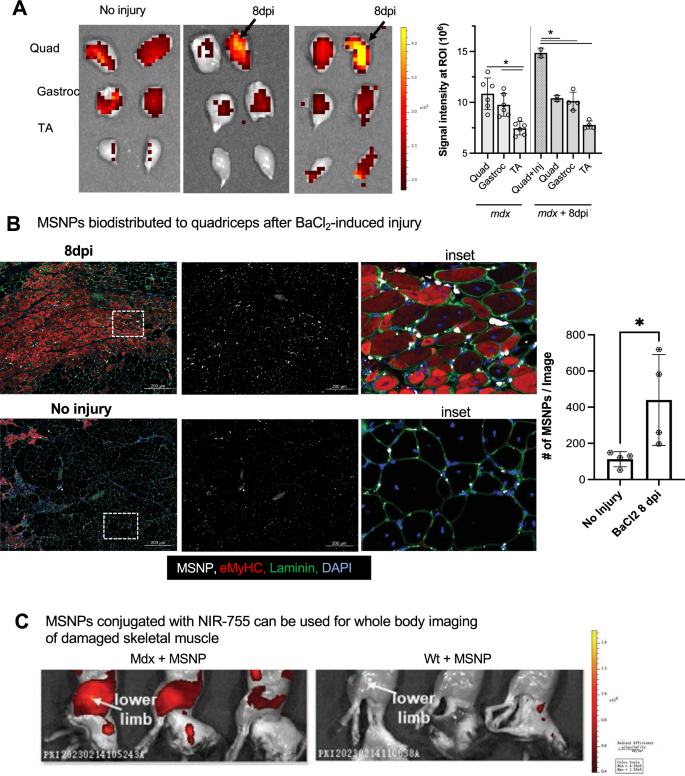
We reasoned that if MSNPs biodistributed to websites of muscle regeneration, then we should always be capable to induce muscle damage and establish MSNPs gathered at websites of regeneration. Barium chloride (BaCl2) is a generally used agent which robustly induces skeletal muscle regeneration following native intramuscular injection [42]. Instantly following injection, BaCl2 blocks calcium channels of skeletal myofibers leading to myofiber demise, native irritation, and blood vessel fragmentation inflicting non permanent ischemia [43]. The most recent regenerating myofibers generated from satellite tv for pc cell differentiation happen roughly 7 days after damage [44]. To validate whether or not we may induce a regenerative response with corresponding blood vessel restoration, we carried out BaCl2 injections and evaluation in skeletal muscle of unhurt, 1 day, and seven days publish damage (dpi). At 7 dpi, we recognized excessive numbers of α-SMA + eMyHC + myofibers juxtaposed to dilated endothelial cells marked by Cd31 + α-SMA-, in addition to an inflow of Cd68 + macrophages juxtaposed to eMyHC + myofibers (Further file 4: Fig. S4A-B). To indicate purposeful vascular perfusion at the moment level, we carried out the Miles assay utilizing Evan’s Blue dye (EBD) which permits researchers to check vascular hyperpermeability by way of the proxy measurement of vascular leakage [45], and confirmed an elevated EBD replace 1 h after intravenous injection relative to unhurt controls (Further file 4: Fig. S4C).
After validating muscle regeneration and endothelial cell permeability at the moment level, we injected a single quadriceps website with BaCl2 and after 7 dpi, delivered NIR-labeled MSNPs to exhibit diagnostic detection and bio-distribution at regenerative websites. In keeping with our earlier knowledge, non-injured mdx mice had sturdy sign in quadriceps and gastrocnemius, and NIR sign was equally distributed between left and proper legs of unhurt mdx mice (Fig. 4B). Nevertheless, in BaCl2 handled quadriceps, the MSNPs confirmed a major enhance in sign relative to different non-injured muscle tissues (p < 0.05, Fig. 4B-C). NIR signaling knowledge was additional validated by immunofluorescent staining which confirmed massive numbers of MSNPs interspersed between endothelial cells and laminin basal membrane of eMyHC + myofibers (Fig. 4B). To guage whole-body distribution of MSNPs in wild sort and dystrophic mice we synthesized NIR Dylight 755 labeled MSNPs which have decrease background interference for dwelling animals imaging. We carried out the whole-body imaging of the sacrificed animal with hair elimination at 24 h post-injection, which demonstrated the nanoparticles selectively gathered on the muscle groups in each higher and decrease limbs in mdx mice however not in wildtype C57 mice (Fig. 4C).
MSNPs biodistributed to quadriceps after experimental-induced damage. A. MSNPs had been injected 7 days publish damage to proper mdx quadriceps. Ex vivo NIR fluorescence imaging of limb muscle groups at 24 h after animals acquired IV injection of NIR-labeled lipid-coated 70 nm MSNPs (50 mg/kg). B. Tissue sections and graph present enhance accumulation of MSNPs (white) close to eMyHC (crimson) optimistic myofibers. Graph quantifies, the scale and fluorescent depth of MSNPs in BaCl2-injured and non-injured mdx quadriceps (N = 3). C. Complete physique imaging of animals at 24 h after IV injection of DyLight755 labeled MSNPs