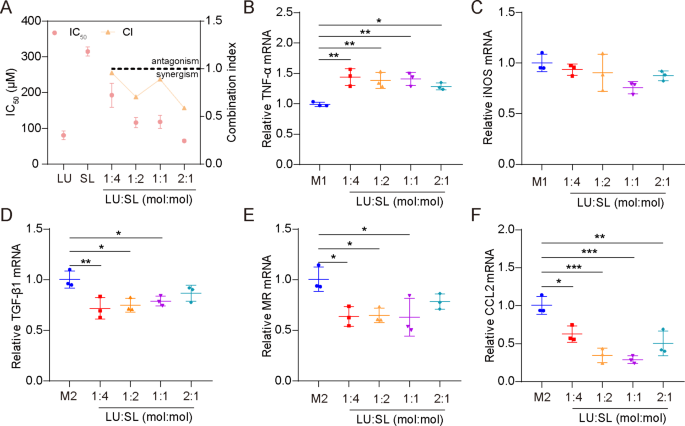
Optimum ratio of LU and SL together
The synergistic motion of LU and SL have been evaluated primarily based on cell viability of 4T1 cells and mRNA expression of M1/M2 repolarized Raw264.7 cells. All molar ratio mixtures (4:1, 2:1, 1:1 and 1:2) of LU and SL confirmed a mixture index (CI) < 1 (Fig. 1A), demonstrating synergistic results with elevated tumor cytotoxicity [27]. Moreover, the mix of LU and SL upregulated M1-related mRNA TNF-α (Fig. 1B) or maintained the iNOS stage (Fig. 1C) and exhibited an optimized impact on the re-education of M2 macrophages to M1 macrophages, mirrored by downregulated M2-related mRNA TGF-β1, MR, and CCL2 (Fig. 1D-F). LU and SL in a 1:1 molar ratio confirmed favorable synergistic inhibitory results with a CI of 0.889 in addition to optimized macrophage repolarization results. Subsequently, the molar ratio of 1:1 was used because the optimum ratio to manufacture SDPN on this research.
Optimum ratio of a mixture of LU and SL, as decided by cell viability and qPCR assays. (A) The cytotoxicity (IC50) and mixture index (CI) in 4T1 cells with totally different ratios of free LU/SL. (B-F) The relative mRNA expression of the macrophage-associated markers TNF-α, iNOS, TGF-β1, MR, and CCL2 by qPCR assay after LU/SL remedy in Raw264.7 cells. Imply ± SD, n = 3; *P < 0.05, **P < 0.01, ***P < 0.005. Abbreviations: LU/SL, luteolin and silibinin; SD, commonplace deviation; qPCR, quantitative polymerase chain response
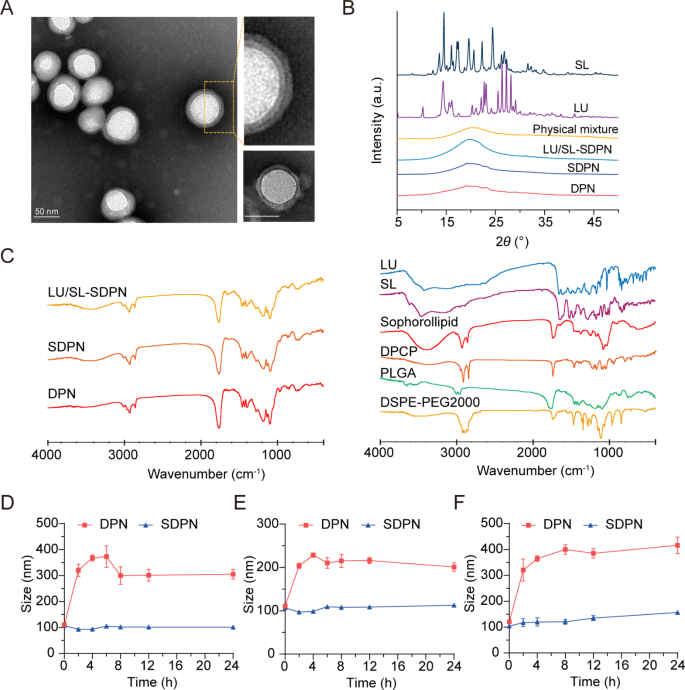
Characterization of SDPN
We fabricated cell membrane-biomimetic lipid polymeric hybrid nanoparticles SDPN by a modified nanoprecipitation technique utilizing DPCP and DSPE-MPEG2000 hooked up to sophorolipids after which evaluated the nanoparticles. The obtained SDPN have been lower than 100 nm in measurement (Desk 2). They confirmed a decrease absolute zeta potential than the nanoparticles with out sophorolipid attachment (DPN). The lipid was coated onto the PLGA core to type the shell-core construction. Because the lipid shell consists of DPCP, the thickness of DPCP on the floor of PLGA is related. The encapsulation effectivity of LU and SL in SDPN have been 97.1% and 96.5%, respectively, whereas their drug loadings have been 3.49% and a couple of.04%, respectively. Attributable to their hydrophobic interactions, LU and SL may largely be loaded into PLGA core, and a number of other of them have been distributed on the interface of lipid DPCP and PLGA of SDPN. The drug loading of SDPN is especially associated to the quantity of PLGA; the extra PLGA, the extra hydrophobic medication are loaded. Moreover, the lipid shell is coated on the core floor of PLGA to additional shield the drug from leaking out. SDPN displayed a spherical form with an apparent core-shell construction and exhibited each a uniform particle measurement distribution and a uniquely-arranged outer layer (Fig. 2A). This was in step with earlier characterizations of affiliation between nanoparticle surfaces and sophorolipids [7]. The outcomes of XRD are proven in Fig. 2B. The XRD spectrum of SL exhibited sturdy crystal diffraction peaks at 13.06°, 16.08°, 17.44°, 19.6°, 20.26°, 22.28°, and 24.42°, whereas LU confirmed peaks at 10.26°, 13.5°, 22.12°, 23.1°, 25.54°, 26.36°, 27.24°, and 28.14°, indicating the crystal type of the 2 medication. Nonetheless, these crystal diffraction peaks disappeared within the patterns of clean DPN, SDPN, and LU/SL-SDPN, indicating the modified crystal state of SL and LU in nanoparticles, which can be molecular or amorphous varieties in SDPN. Additional, the attainable physicochemical interactions between the elements in nanoparticles in addition to the therapeutic agent loading have been evaluated by FTIR evaluation. As proven in Fig. 2C, the sharp and huge peak at 1760 cm− 1 within the FTIR spectra of DPN and SDPN may very well be ascribed because the C = O stretching vibration overlapping of the peaks of PLGA, DPCP, and DSPE-PEG2000. The peaks at 1455 cm− 1 and 1135 cm− 1 associated to methyl group C-H stretching and C-O-C stretching, respectively, may belong to PLGA. The height at 2920 cm− 1 noticed within the DPN spectrum is likely to be described because the methylene group C-H of DPCP. In contrast with the DPN spectrum, the stronger peak at 3420 cm− 1 of the SDPN spectrum may belong to the precise O-H vibration of sophorolipids. That is proof that sophorolipids are hooked up on the floor of SDPN. Encapsulation of luteolin and silibinin have been confirmed because of their particular peaks at 1520 cm− 1 (fragrant ring), 1610 cm− 1 (C = O from central heterocyclic ring), and 3430 cm− 1(OH) weakening or disappearing within the LU/SL-SDPN spectrum. The gastrointestinal tract is a fancy physiological atmosphere, and components similar to pH, digestive enzymes, ions, and endogenous substances will have an effect on the soundness of nanoparticles [1]. Subsequently, guaranteeing the excessive stability of nanoparticles within the gastrointestinal tract is the first precondition for oral absorption. There was no vital particle measurement change for SDPN, indicating that SDPN remained steady in SGF (Fig. 2D) and SIF (Fig. 2E). Moreover, SDPN additionally confirmed stability in PBS at 37℃ (Fig. 2F). In step with our earlier research [7], the attachment of sophorolipids shaped a protected layer because of their distinctive function of resisting pH and ion energy variation.
Nanoparticle characterization and nanoparticle bodily stability in simulated gastrointestinal fluids. (A) Transmission electron microscope picture of SDPN (left) and DPNs (decrease proper nook); Scale bar = 50 nm. (B) X-ray diffraction patterns of SL, LU, bodily combination, drug-free DPN, SDPN, and LU and SL co-loaded SDPN. (C) FTIR spectra of LU/SL-SDPN, SDPN, DPN (left) and luteolin, silibinin, sophorolipid, DPCP, DSPE-PEG2000, and PLGA (proper). (D, E) Imply particle measurement of nanoparticles in simulated gastric fluid (D), simulated intestinal fluid (E), and PBS (pH7.4) (F). Imply ± SD, n = 3. Abbreviations: SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; LU, luteolin; SL, silibinin; FTIR, Fourier-transformed infrared; PLGA, poly(lactic-co-glycolic acid)
In vitro cytocompatibility and hemolysis assay
The cytotoxicity was evaluated to review the in vitro cytocompatibility of DPN and SDPN. The cell viability of DPN at every focus was above 95% (Fig. S1A). For SDPN, the cell viability was 95.1%, 94.6%, and 90.3% on the concentrations of 0.1, 0.5, 1.0 mg/mL, respectively (Fig. S1B). These outcomes demonstrated the favorable cytocompatibility of DPN and SDPN in vitro.
The hemolysis share (HP) of DPN and SDPN have been decided to guage nanocarrier blood compatibility. The HP of DPN was decrease than 3.5%, which confirmed a big lower in comparison with the constructive management group (H2O) (Fig. S2A). The HP of SDPN was decrease than 3%, additionally exhibiting a big lower with the constructive management group (Fig. S2B). The HP was in accordance with the permissible stage of 5%, which indicated their negligible hemolysis and favorable security for in vivo research.
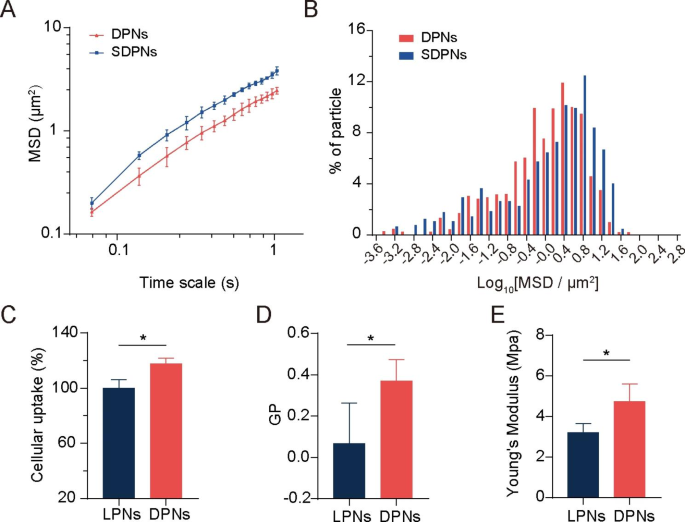
Mucus diffusion and in vitro mobile uptake
We evaluated the mucus diffusion of nanoparticles via multiple-particle monitoring assays. The bigger imply sq. displacement (MSD) worth (Fig. 3A) and better share of particles with excessive mobility (Fig. 3B) exhibit the great mucus permeation potential of SDPN. SDPN has hydrophilic and near-neutral floor properties as a result of attachment of sophorolipids that enhance their mucus penetration capability. Moreover, sophorolipid assemblies might dissociate from the floor of nanoparticles throughout mucus transit because of their weak interplay with the outer lipid fraction of nanoparticles, in response to our earlier research [7], which permits the publicity of DPCP to generate endocytosis by enterocytes.
Mucus diffusion and mobile uptake research. (A) Imply sq. displacement (MSD)–time interval curve for the nanoparticles. (B) Histogram of MSD distribution of nanoparticles on a 1.035-s time scale. (C) Mobile uptake of LPN and DPN in Caco-2 cells. (D) Generalized polarization (GP) worth of LPN and DPN. (E) The Younger’s modulus of LPN and DPN. Imply ± SD, n = 3; *P < 0.05. Abbreviations: SD, commonplace deviation; LPN, lipid-polymer nanoparticles; DPN: choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle
The membrane-biomimetic technique was anticipated to enhance the mobile uptake capability of DPN. To research this speculation and consider the advantages of the cell membrane interplay of DPCP to nanoparticles uptake, we used nanoparticle LPN with PC cowl as an alternative of DPCP as a management. In contrast with LPN, DPN considerably improved the mobile uptake fee in Caco-2 cells (Fig. 3C), indicating the next internalization effectivity. Cell membrane phospholipids include a zwitterionic PC head. DPCP, as reported in a earlier research [2], was synthesized with three elements: headgroups (hydrophilic head composed of PC-reversed choline and phosphate teams), a C16 alkane tail, and a linker. The synthesized hydrophilic head in DPCP conferred a cost course reversal and was thought-about to supply biorthogonality within the spine. In a earlier research [24], CP exhibited cell adhesion motion and protein resistance because of its pure zwitterionic properties and supramolecular ionic pair interactions with cell membranes. Within the present research, as illustrated in Scheme 1 A, when DPN (accessible publish SDPN mucus diffusion) contacted an enterocyte, the CP-PC interplay might have occurred by way of supramolecular ion pair (-(CH3)3 N+—PO3−) with PO3−—(CH3)3 N+). The interplay with cell membranes via CP-PC promoted the adhesion of nanoparticles to enterocytes and improved the affinity of nanoparticles with cell membranes, resulting in environment friendly nanoparticle cell internalization. The biomimetic utility of DPCP in SDPN supply offered a common binding mechanism to connect nanoparticles to enterocyte membranes. A number of components additionally affect the uptake conduct and effectivity of nanocarriers [28], similar to measurement, floor properties, fluidity, rigidity, and ligand properties of floor modifications. As DPN and LPN have related floor expenses and particle sizes, it advised that the scale and cost of nanoparticles may not be the principle causes of their variations in mobile uptake. Thus, we measured the fluidity of nanoparticle membrane containing lipids. The GP values of DPN have been considerably greater than these of LPN (Fig. 3D), demonstrating that DPN have been much less fluid in contrast with LPN. The floor fluidity of lipids has been confirmed to facilitate interplay with cells because of elevated alternatives for nanoparticles to contact and fuse with cell membranes [29]. When nanoparticles are endocytosed, they encounter two opposing forces. One is the enticing pressure driving nanoparticles and cells collectively, comprising van der Waals forces, electrostatic motion, and ligand-receptor interactions. The opposite is a repelling pressure that stops nanoparticles from getting into the cell, affecting wrapping of nanoparticles, bending of the cell membrane, and membrane stress. Nonetheless, the better fluidity of the nanoparticle lipid layer means extra power is required to beat the bending change within the cell membrane in addition to the encapsulation of the nanoparticles, facilitating internalization of extra fluid nanoparticles. The bigger the wetting angle of the nanoparticles, the better the extent of nanoparticles unfolding on the floor of the cell membrane wanted [30]. The bending of power boundaries that have to be overcome is said to the extent of the nanoparticles’ unfold. Appropriate membrane fluidity can steadiness these two opposing forces, which is conducive to maximizing internalization effectivity. Subsequently, DPN possessed extra favorable lipid layer fluidity than LPN. We additionally measured the Younger’s modulus of nanoparticles by atomic pressure microscopy to estimate the particles’ general rigidity. The Younger’s modulus of DPN was considerably greater than that of LPN (Fig. 3E), indicating that DPN may keep inflexible. Deformation just isn’t obvious when getting into the cell membrane, partly explaining its accessibility for mobile internalization [25]. It has been proven that nanoparticles with average rigidity not solely exhibit enhanced diffusion in mucus, but in addition overcome the intestinal barrier [31]. Tender nanoparticles exhibit weak mucus penetration and low mobile uptake because of their extreme change and irregular form. Therefore, spherical nanoparticles with a sure stiffness are inclined to deform into ellipsoids in a fancy mucus community construction, selling fast penetration and exhibiting higher mobile uptake functionality. The acceptable fluidity and general rigidity of nanoparticles is likely to be nicely offered by the DPCP because the lipid element of DPN, affecting the nanoparticle floor property and being useful to mobile uptake.
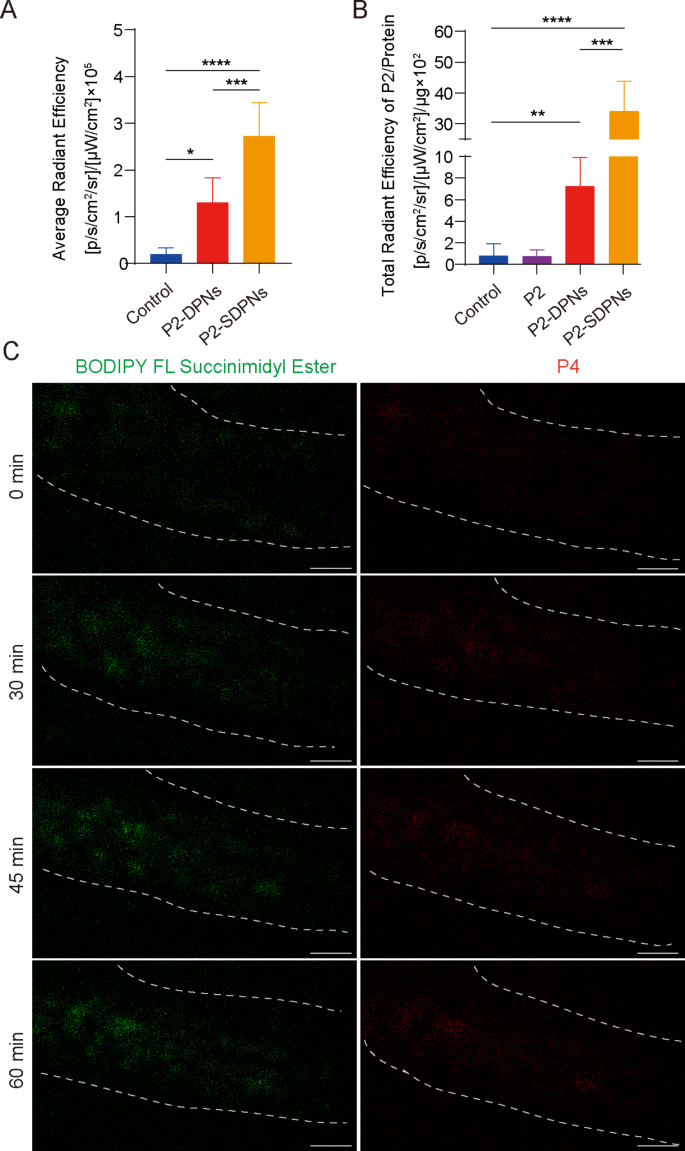
In vivo intestinal absorption and transport
The destiny of SDPN within the gastrointestinal tract was additional revealed by detecting the in vivo intestinal absorption and in situ Peyer’s patch absorption in addition to the switch into the mesenteric vascular system. To trace intact nanoparticles labeled with fluorescent probes, a P2 probe was used for nanoparticle oral intestinal absorption research. P2 is a category of near-infrared fluorescence aza-BODIPY probes, emitting steady and powerful fluorescence when carried in hydrophobic cores of nanoparticles [32, 33]. In contrast with DPN, the fluorescence depth of SDPN was considerably elevated (Fig. 4A), indicating that SDPN improved absorption by the intestinal epithelium, in step with the short permeation via the mucus layer and improved cell uptake in vitro. Peyer’s patch absorption of SDPN in rats was additionally considerably elevated (Fig. 4B), which was anticipated for the lymphatic pathway absorption of nanoparticles. Moreover, the transport of SDPN into mesenteric vessels was noticed by two-photon microscopy in situ in rats. SDPN are loaded with the fluorescent probe BODIPY FL Succinimidyl Ester (inexperienced sign) within the related sophorolipid and P4 (pink sign) within the core. SDPN confirmed distinct fluorescence in mesenteric vessels at 30, 45, and 60 min (Fig. 4C) in comparison with 0 min, suggesting {that a} portion of the nanoparticles have been transported intact via the mesenteric vessels and absorbed into the blood.
Nanoparticle intestinal and Peyer’s patch absorption and mesenteric vascular transport in situ within the rat. (A) Fluorescence depth evaluation of DPN and SDPN in jejunum after oral administration for 1 h. (B) Fluorescence depth of DPN and SDPN per unit of Peyer’s patch protein after oral administration for 1 h. Imply ± SD, n = 3; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) Transportation of SDPN into mesenteric vascular tissue after administration for 0, 30, 45, and 60 min imaged by two-photon microscopy. The mesenteric blood vessel space was circled by the dotted line. Scale bar = 100 μm. Abbreviations: SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; SD, commonplace deviation
In vitro regulation of macrophage polarization
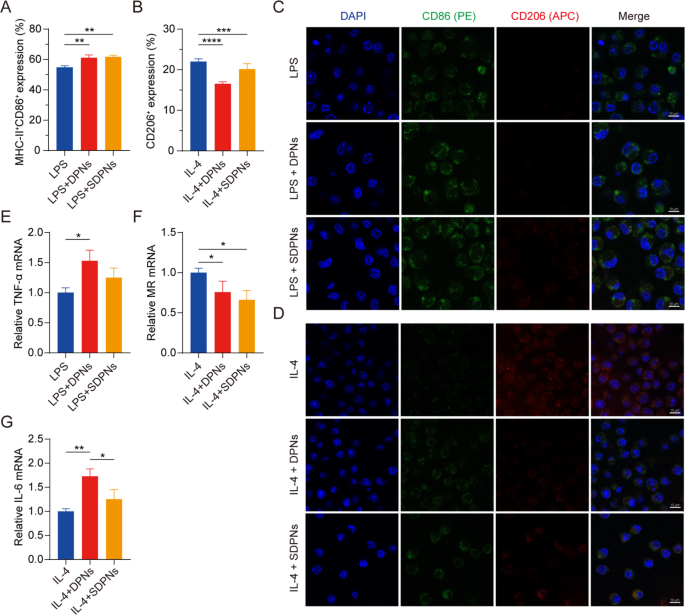
To research the position of co-loaded nanoparticles in regulating M1 and M2 macrophage polarization, we evaluated the expression of the macrophage protein biomarkers CD86, MHC-II, and CD206 by movement cytometry with M1 or M2 RAW264.7 cells, induced by LPS or IL-4, respectively. The expression of CD86+ MHC-II+ within the LPS + DPN and LPS + SDPN teams was considerably elevated in contrast with the LPS group (Fig. 5A), indicating that the nanoparticles might promote M1 phenotype macrophages. The CD206+ expression within the IL-4 + DPN group decreased considerably in contrast with the IL-4 induction group (Fig. 5B), indicating that nanoparticles might suppress M2 macrophages. As well as, in contrast with the IL-4 + SDPN group, the expression of CD206+ within the IL-4 + DPN group was decrease, which is likely to be as a result of totally different floor properties of nanoparticles giving rise to totally different macrophage regulation mechanisms or mobile uptake talents [34].
In vitro regulation of macrophage repolarization by nanoparticles in Raw264.7 cells. (A, B) Circulate cytometry measurement of MHC-II+CD86+ or CD206+ expression after remedy with nanoparticles in M1 macrophages (300 ng/mL LPS induction) or nanoparticles in M2 macrophages (60 ng/mL IL-4 induction). (C, D) Laser confocal picture of nanoparticles on M1/M2 macrophage. Blue fluorescence: cell nuclei by DAPI; Inexperienced fluorescence: CD86; Crimson fluorescence: CD206. Scale bar = 10 μm. (E-G) The relative mRNA expression of the macrophage-associated markers TNF-α, MR, and IL-6 by qPCR assay. Imply ± SD, n = 3; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. Abbreviations: qPCR, quantitative polymerase chain response; SD, commonplace deviation; DAPI, 4′,6-diamidino-2-phenylindole; LPS, lipopolysaccharide
As proven within the CLSM photographs (Fig. 5C and D), SDPN elevated inexperienced fluorescence sign depth (CD86) within the in vitro M1 macrophage mannequin induced by LPS, whereas the pink sign depth (CD206) remained unchanged. In M2 macrophages, SDPN remarkably decreased the pink fluorescence depth whereas usually rising the inexperienced one. In step with the movement cytometry outcomes, the CLSM observations demonstrated that SDPN upregulated M1-related CD86 ranges and downregulated M2-related CD206 ranges.
In qPCR assays, Fig. 5E and F present that the repolarization impact of the nanoparticles is demonstrated by the notably elevated ranges of the M1-related anti-tumor cytokine TNF-α, whereas decreased relative mRNA ranges of the M2-related biomarker MR. As well as, the expression of IL-6 was upregulated (Fig. 5G); this upregulation is said to M1 macrophages, indicating the repolarization of M2 to the M1 macrophage phenotype. Collectively, these findings advised that DPN and SDPN regulated the Raw264.7 macrophage phenotype, selling M1 phenotype macrophages and lowering M2 phenotype macrophages, and implying that there was a polarization impact of nanoparticles on TAM from the tumor-promoting to the anti-tumor phenotype.
In vitro regulation of OXPHOS and glycolysis
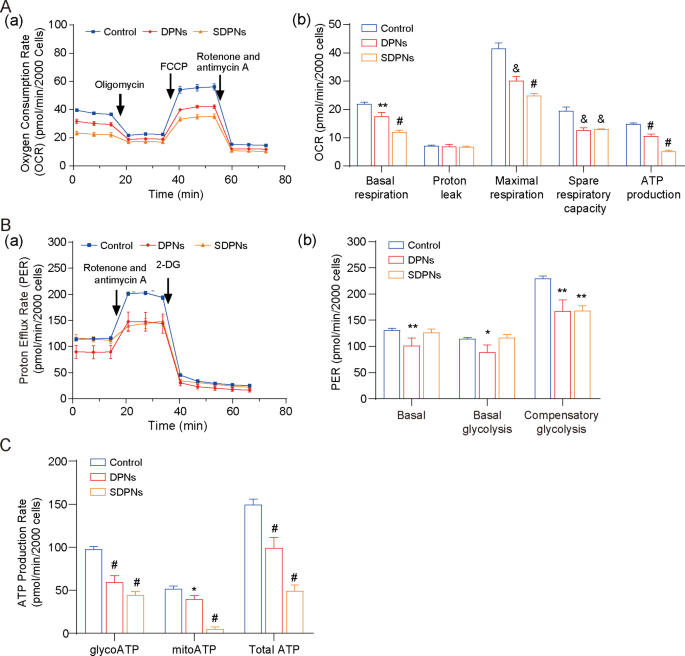
4T1 is a rapidly-proliferating and extremely invasive cell line that displays excessive metabolic plasticity (metabolic reprogramming potential), simply switching between OXPHOS and glycolysis to coordinate the manufacturing of ATP and the availability of biosynthetic precursors in response to environmental stress [35]. For the investigation of LU/SL-SDPN affecting the metabolic adjustment in breast most cancers cells, the hypoxic 4T1 cell mannequin was used. The OCR of 4T1 cells handled with SDPN (Fig. 6A) have been considerably decreased versus management, confirming that DPN and SDPN might considerably inhibit oxidative phosphorylation (OXPHOS) in 4T1 cells. As well as, DPN considerably lowered the proton efflux fee (PER), together with basal PER, glycoPER, and compensatory glycolysis PER (Fig. 6B). SDPN lowered the compensatory glycolysis PER. These outcomes advised that SDPN might inhibit glycolysis in 4T1 cells. Moreover, SDPN considerably inhibited mitoATP and glycoATP manufacturing (Fig. 6C). Collectively, SDPN inhibited the power metabolism of 4T1 cells by inhibiting each glycolysis and OXPHOS, implying that the regulation of metabolic power offered help to breast most cancers metastasis.
In vitro nanoparticle regulation of metabolism in 4T1 cells below hypoxic circumstances: (A) Mito stress take a look at. (a) OCR curves in 4T1 cells handled with oligomycin, FCCP, and each rotenone and antimycin A. (b) OCR values of basal respiration, most respiration, spare respiratory capability, and ATP manufacturing handled with LU/SL-DPNs and LU/SL-SDPNs for 1 h. (B) Glycolytic fee take a look at. (a) PER curves in 4T1 cells handled with 2-DG and each rotenone and antimycin A. (b) PER values of basal proton efflux fee, glycolytic proton efflux fee, and compensatory glycolysis handled with LU/SL-DPNs and LU/SL-SDPNs for 1 h. (C) Actual-time ATP take a look at for glyco ATP, mito ATP, and whole ATP handled with LU/SL-DPNs and LU/SL-SDPNs for 1 h. (imply ± SD, n = 3) In contrast with the management group. *P < 0.05, **P < 0.01, &P < 0.005, #P < 0.001. Abbreviations: OCR, oxygen consumption fee; SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; qPCR, quantitative polymerase chain response; SD, commonplace deviation; LU/SL, luteolin and silibinin
In vivo antimetastatic impact
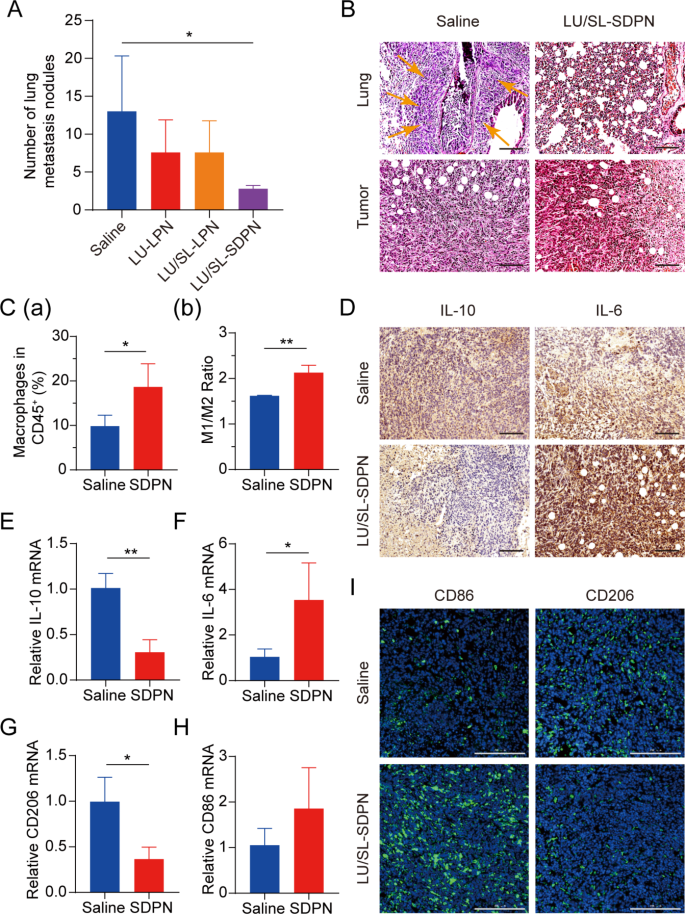
Balb/c mice bearing 4T1 tumor cells have been used to evaluate the results of LU/SL-SDPN in vivo on the alleviation of breast most cancers development and metastasis. The LU/SL-SDPN-treated group had a considerably lowered variety of metastatic pulmonary nodes in contrast with the saline-treated group (P < 0.05) (Fig. 7A). Furthermore, H&E staining of lung tissue demonstrated the plain intrapulmonary metastasis of malignant tumor cells with destroyed alveoli within the saline-treated group, whereas the LU/SL-SDPN-treated group confirmed thickened alveoli septa (Fig. 7B). The outcomes of H&E staining of tumor tissues from the saline-treated group confirmed that tumor cells invaded and infiltrated surrounding tissues, however tumor tissues of the LU/SL-SDPN-treated group have been partly necrotic and confirmed inflammatory cell infiltration. These outcomes indicated that LU/SL-SDPN alleviated breast most cancers lung metastasis.
Antimetastatic exercise of nanoparticles in vivo. (A) Variety of lung metastasis nodules from 4T1 tumor-bearing mice in every remedy group. (B) H&E staining of tumor and lung tissue sections. Arrows level to the world of intrapulmonary metastasis of tumor cells. Scale bar = 100 μm. (C) Evaluation of macrophages in 4T1 tumors by movement cytometry. Whole macrophages proportion (a) and M1/M2 ratios (b) of saline and SDPN group-treated tumor tissues. (D) Immunohistochemical staining of IL-10 and IL-6 in tumor tissue sections of saline and SDPN teams. Scale bar = 100 μm. (E-H) The relative mRNA expression of the macrophage-associated markers IL-10, IL-6, CD206, and CD86 in tumors by qPCR assay. (I) Immunofluorescence staining of tumor CD86 and CD206 in tissue sections. Scale bar = 200 μm. Imply ± SD, n = 3; *P < 0.05, **P < 0.01. Abbreviations: SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; qPCR, quantitative polymerase chain response; SD, commonplace deviation; H&E, hematoxylin and eosin
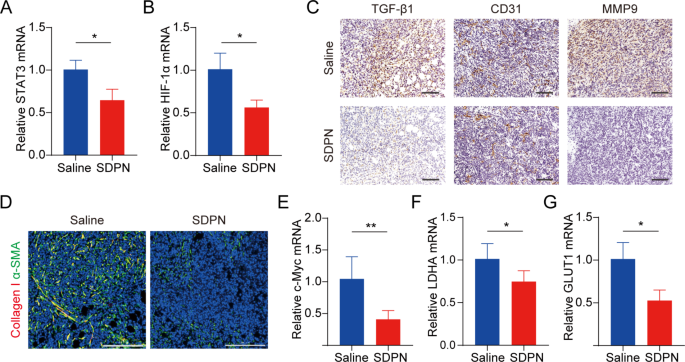
We evaluated LU/SL-SDPN regulation of TAM by analyzing the associated protein expression in tumor tissues to disclose the underlying mechanism. In contrast with the saline group, macrophage (F480+CD11b+) proportion in whole lymphocytes elevated by 8.8% (Fig. 7C(a)). The ratio of the variety of MHC-II+ cells to CD206+ cells within the LU/SL-SDPN-treated group (i.e., the M1/M2 ratio) considerably elevated (Fig. 7C(b), S3). Constantly, immunohistochemical staining (Fig. 7D), qPCR (Fig. 7E-H), and immunofluorescence staining (Fig. 7I, S4A) ends in the LU/SL-SDPN-treated group confirmed upregulation of the M1-related cytokine IL-6 and biomarker CD86 and downregulation of M2-related IL-10 and CD206. Collectively, these outcomes indicated that LU/SL-SDPN promoted the M1 phenotype and inhibited the M2 phenotype, thereby regulating TAM to M1-like macrophage polarization and bettering anti-tumor-metastasis results. The technique of regulation of TAM to M1 macrophages exploits the affect of M1 macrophages on tumor inhibition, metastasis, and immune activation, by presenting particular protein and secreting cytokines in TME whereas lowering macrophage elimination. LU has proven the potential to manage macrophage polarization. LU lowered gene expression in M2 macrophages by inhibiting the phosphorylation of STAT proteins [36]. STAT3 is a crucial intrinsic pathway that intervenes with TAM polarization [26, 37]. With the elimination of the STAT3 signaling pathway, TAM polarization to the M2 phenotype was lowered, thus inducing anti-tumor immunity [38]. Moreover, tumor hypoxia impacts macrophage polarization. The upregulation of HIF1-α in tumor hypoxia contributes to the next distribution of M2 macrophages, selling angiogenesis. Constantly, LU has been reported to inhibit VEGF expression in M2-like TAM and to inhibit activation of HIF1-α and STAT3 phosphorylation sign transduction below hypoxic circumstances [19, 36]. Herein, the expression of STAT3 and HIF1-α was considerably downregulated (Fig. 8A-B), suggesting the therapeutic potential of LU/SL-SDPN via the co-action of STAT3 and HIF1-α inhibition, to alleviate breast most cancers lung metastasis by lowering the reset of M2 macrophages and rising M1 macrophages.
TME and metabolism regulation in 4T1 tumor-bearing mice handled with SDPNs. (A, B) The relative mRNA expression of STAT3 and HIF1-α in tumor tissues by qPCR assay. (C) Immunohistochemical staining of TGF-β1, CD31, and MMP-9 in tumor tissue sections of saline and SDPN-treated teams. Scale bar = 100 μm. (D) Immunofluorescence staining of tumor tissue sections for collagen I and α-SMA. Scale bar = 200 μm. (E-G) The relative mRNA expression of c-Myc, LDHA, and GLUT1 in tumor tissues by qPCR assay. Imply ± SD, n = 3; *P < 0.05, **P < 0.01. Abbreviations: TME, tumor microenvironment; SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; qPCR, quantitative polymerase chain response; SD, commonplace deviation
It has been reported that TAM participates in regulating the EMT course of, promotes angiogenesis, and degrades the extracellular matrix with MMP and different pathways, thus selling tumor metastasis [39, 40]. Subsequently, we additional investigated the modifications in EMT, angiogenesis, and the ECM handled with SDPN. LU/SL-SDPN was proven to have downregulated the expression of TGF-β1, CD31, and MMP9 by immunohistochemistry staining (Fig. 8C), indicating that LU/SL-SDPN might suppress cancer-associated fibroblasts, alleviate the EMT, and scale back angiogenesis and ECM degradation to manage the TME. As well as, LU/SL-SDPN downregulated the fluorescence colocalization expression of collagen I and α-SMA in tumor tissue (Fig. 8D, S4B), indicating that the nanoparticles might to some extent scale back fiber formation on the tumor website to inhibit the matrix barrier, serving to enhance the penetration and accumulation of nanoparticles in tumor tissues and additional bettering inhibition of breast most cancers metastasis. Aside from the contribution of TAM regulation, SL impacts the regulation of TME and hampers breast most cancers invasion and metastasis [8, 41]. SL has additionally been proven to inhibit angiogenesis [42] and the cytokine TGF-β1[43]. The TGF-β pathway is carefully associated to the activation of most cancers, which boosts profibrotic signaling transduction and modifications the ECM composition, constituting a fancy matrix atmosphere in tumor tissues [44], whereby extreme collagen fibers interlace to type a reticular barrier, which can isolate immune cells from the tumor tissue, whereas additionally affecting drug penetration and supply [45].
mRNA expression of c-Myc, GLUT1, and LDHA have been downregulated in LU/SL-SDPN-treated tumor tissues (Fig. 8E-G), in step with the fluorescence depth of the GLUT1 protein, proven in Fig. S4C; this implies the downregulation of OXPHOS and glycolysis metabolism-related gene expression. Mitochondrial STAT3 promotes OXPHOS via the electron transport chain, whereas STAT3 within the nucleus can promote cardio glycolysis, despite the fact that the enhancement of glycolysis just isn’t solely influenced by STAT3 but in addition by HIF1-α and the proto-oncogene MYC [46]. c-Myc transcription issue, one of many merchandise of HIF-1α and Myc, regulates cardio glycolysis of tumor cells by rising the transcription of widespread targets, similar to GLUT1 and LDHA [47]. The inhibition of the overexpression of STAT3, HIF-1α, c-Myc, GLUT1, and LDHA indicated that SDPN can inhibit breast most cancers metastasis by concentrating on the potential indicators talked about above.
H&E staining outcomes confirmed no vital organ abnormalities of the liver, coronary heart, spleen, kidney, and jejunum within the saline or LU/SL-SDPN-treated teams, apart from the presence of tumor cell metastases within the liver, which was anticipated with the tumor-bearing mice mannequin. Furthermore, SDPN didn’t result in in vivo organ toxicity, indicating the histological security of SDPN (Fig.S5).
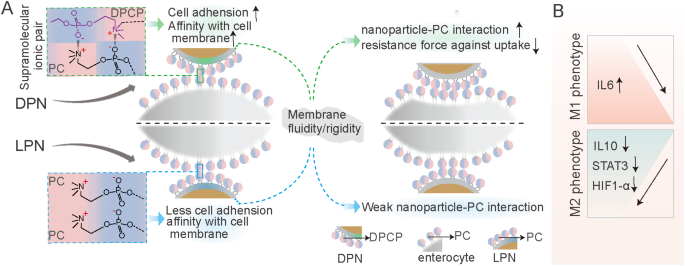
The present research demonstrated that SDPNs confer advantages in enhancing endocytosis and the regulation of TAM (Fig. 9). The endocytosis promotion is summarized as follows: when DPN accessible on the floor of enterocyte (after SDPN transferred via the mucus layer), the supramolecular interplay offered distinctive cell adhesion and affinity with the cell membrane. Then, the modified membrane fluidity and rigidity of DPNs favored the DPCP-PC interplay, and presumably lowered the resistance pressure towards endocytosis. The improved oral supply effectivity of the bioactive substances of LU and SL enhanced the motion in regulation of TAMs polarization. LU/SL-SDPNs upregulated cytokines IL-6 and downregulated IL-10 in tumor tissues. Moreover, LU/SL-SDPNs inhibited the motion of STAT3 and HIF1-α, which have been thought-about to be the necessary transcription components concerned in M2 polarization.
Schematic exhibiting the marketing endocytosis of DPN accessible on the floor of enterocyte (after SDPN transferred via the mucus layer) (A); regulation of tumor-associated macrophage polarization handled with luteolin- and silibinin-co-loaded SDPN (B). Abbreviations: SDPN, sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle; DPN: choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle