Herein, we studied the traits of the ready nano-formulation (DIP-FU-PPy NPs). We encapsulated an antithrombotic agent, DIP, into this nanocarrier system and evaluated its materials properties, resembling drug-LE, particle dimension, zeta potential, morphological properties, NIR-photothermal properties, in vitro mobile interactions, drug-release habits, and physiochemical properties. We additionally evaluated its capabilities, resembling inhibition of clot formation, antiplatelet aggregation, and clot lysis in vitro, impact of P-selectin-mediated cell accumulation of DIP-FU-PPy NPs, and the mixed therapeutic impact of DIP-FU-PPy NPs. Ferric chloride was used to induce fibrin-insoluble mesenteric thrombi in an in vivo ICR mouse mannequin. When reaching the goal thrombus web site after systemic administration, the mixed therapeutic impact of non-invasive NIR nano-photoablation and the discharge of therapeutic DIP was anticipated to behave in opposition to the thrombus, leading to focused loosening with decision of the shaped clot. We hypothesized that the focused therapeutic mechanism of DIP-FU-PPy NPs concerned exact drug supply with phototherapeutic behaviors, thrombus-targeting and penetration efficiency, and secure and environment friendly thrombus administration, which have been validated utilizing biochemical, histological, and in vivo examinations.
Materials preparation and physicochemical characterization
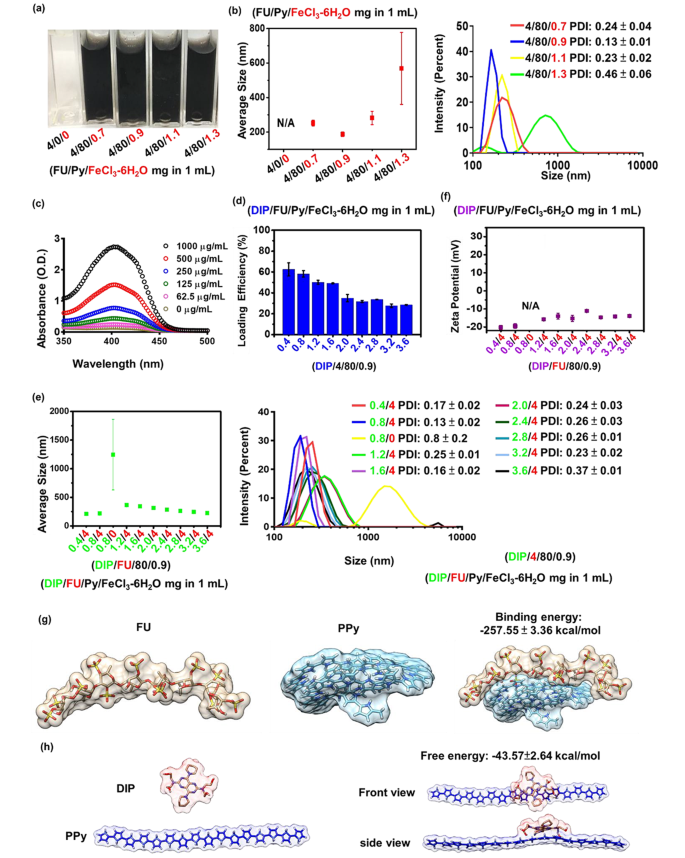
A small dimension (10–300 nm) of hydrophilic NPs is a typical prerequisite for the profitable supply for biomedical use [36]. Hydrophobic PPy could be synthesized through aqueous chemical oxidative polymerization within the presence of ferric ions. Throughout PPy polymerization, mixing FU with an anionic amphiphilic construction stabilizes the PPy. As proven in Fig. 1a, underneath the identical feed concentrations of 4 mg/mL FU, photographic remark of the answer appeared practically clear earlier than supplementation with the FeCl3-6H2O group. No particle formation was noticed with out supplementation of the FeCl3-6H2O group, whereas particles have been shaped with rising common particle sizes (from roughly 187 to 569 nm) when the FeCl3-6H2O quantity elevated (from 0.9 to 1.3 mg/mL) (Fig. 1b). Based on a earlier examine, PPy formation will increase with rising concentrations of ferric chloride [37]. FU couldn’t stabilize the surplus PPy within the 1.3 mg/mL FeCl3-6H2O group, leading to hydrophobic interactions of PPy-driven aggregation (roughly 569 nm) within the aqueous part. When the quantities of FeCl3-6H2O have been comparatively decrease (at 0.7, 0.9, and 1.1 mg/mL), common particle sizes have been < 300 nm (Fig. 1b). To acquire the smallest nanocarrier, a given quantity of FeCl3-6H2O (0.9 mg/mL) was chosen for additional drug encapsulation.
Within the drug-encapsulation examine, the added hydrophobic DIP was encapsulated in the course of the polymerization of PPy by means of hydrophobic interactions owing to its hydrophobicity. Anionic FU might be able to kind nano-shells across the shaped DIP-PPy. Throughout nano-formation, the core (DIP-PPy)-shell (FU) nanocomplex (DIP-FU-PPy NPs) was generated by self-assembly within the aqueous part. The DIP-LE, as decided by spectrophotometry, confirmed that the DIP absorbance depth elevated in a dose-dependent method, starting from 350 to 460 nm (Fig. 1c). The fragrant construction and π-system of DIP have been answerable for the excessive fluorescence and absorption [38].
The DIP drug-LE decreased with a rise within the DIP dosage as a result of the polymeric payload lacked ample capability to encapsulate the surplus DIP (Fig. 1d) [39]. The DIP/FU/Py/FeCl3-6H2O check teams (0.4/4/80/0.9 and 0.8/4/80/0.9 (mg) per mL answer) confirmed acceptable LEs [40] (DIP LEs of ca. 60%). In line with earlier findings, PPy confirmed an acceptable drug-loading capability [41]. The 0.8/4/80/0.9 DIP/FU/Py/FeCl3-6H2O group exhibited barely bigger particle sizes (~ 220 nm, Fig. 1e) in comparison with these of 0.4/4/80/0.9 DIP/FU/Py/FeCl3-6H2O at ~210 nm, presumably due to the presence of extra DIP current in single NPs within the 0.8/4/80/0.9 DIP/FU/Py/FeCl3-6H2O group. Within the 1.6/4/80/0.9 DIP/FU/Py/FeCl3-6H2O group, its LE was low (49.2%), and particle sizes have been relatively giant (344 nm), in comparison with the 0.8/4/80/0.9 DIP/FU/Py/FeCl3-6H2O group. Thus, 0.8/4/80/0.9 DIP/FU/Py/FeCl3-6H2O was used for subsequent experiments, for which the calculated drug-LC was ~10%. The drug-LC displays the mass ratio of medicine to nanomedicines. Contemplating the restoration end result, it was famous that not the entire Py had been polymerized on this provider system in the course of the synthesis course of.
The particle dimension was decreased from the vary of 1.2/4/80/0.9 to three.6/4/80/0.9 particles (Fig. 1e). Zeta potentials of the ready nano-formulations barely elevated with a rise within the DIP feed quantity (Fig. 1f) owing to contributions from the faintly cationic DIP [42]. In comparison with when FU was current (within the 0.8/4/80/0.9 DIP/FU/Py/FeCl3-6H2O group, roughly 220 nm and − 20 mV), the group missing FU (0.8/0/80/0.9 DIP/FU/Py/FeCl3-6H2O) confirmed an elevated particle dimension to the micro-size vary (above 1000 nm) and no out there zeta potential (N/A) (Fig. 1e, f). In some experimental teams (4/80/1.3 FU/Py/FeCl3-6H2O and 0.8/0/80/0.9 DIP/FU/Py/FeCl3-6H2O), their giant extents of normal deviation of the common particle dimension and better PDI values (> 0.3, as per the left facet distribution information) indicated colloidal instability throughout formulation. These findings confirmed that extra DIP or an absence of Fu stabilization within the provider system resulted in an aggregation or precipitation phenomenon.
This means that FU, as an anionic and amphiphilic surfactant, permits cationic PPy and DIP to shortly co-assemble into steady NPs pushed by hydrophobic ionic forces. This prevented aggregation and enhanced the dispersive stability of NPs by rising the floor cost and electrostatic repulsion or by lowering the interfacial power between the solvent and particles. The molecular dynamic simulation urged that the binding power (-257.55 ± 3.36 kcal/mol) between Fu and PPy and the binding power (-43.57 ± 2.64 kcal/mol) between DIP and PPy might be the rationale behind the self-assembly mechanism (Fig. 1g, h). The molecular degree evaluation additional urged that NPs functionalized with PPy plus Fu have been structurally steady. The molecular dynamic simulations and calculated Fu-PPy binding free power confirmed the experimental outcomes, exhibiting higher binding of the drug to the copolymer.
(a) Photographic observations exhibiting that totally different ratios of pyrrole (Py)/ferric chloride (FeCl3-6H2O) reacted with fucoidan (FU) to optimize the formulation (FU/Py/FeCl3-6H2O 4/80/0.9 mg per mL). (b) Dimension distributions of FU-polypyrrole (PPy) formulations (together with FU/Py/FeCl3-6H2O 4/80/0.9 mg per mL) as measured by dynamic mild scattering (DLS, Malvern Zetasizer, Nano-ZS, ZEN 3600). (c) Spectrophotometric information measured by an UV-Vis spectrophotometer (EPOCH2, BioTek) revealing optical properties of dipyridamole (DIP), whose absorbance depth elevated in a dose-dependent method, starting from 350 to 460 nm. (d) Drug-loading effectivity (LE) and DLS information exhibiting (e) the scale distribution and (f) zeta potential of various formulations (underneath a hard and fast part of FU/Py/FeCl3-6H2O at 4/80/0.9 (mg) per mL). DIP information confirmed that the loading effectivity of DIP decreased with a rise within the DIP dosage as a result of the polymeric payload lacked a ample capability to encapsulate the surplus DIP. (g and h) Molecular dynamic simulation information exhibiting the binding power between FU and PPy or DIP and PPy
Nano-formation, morphological change, and photothermal responsiveness assessments
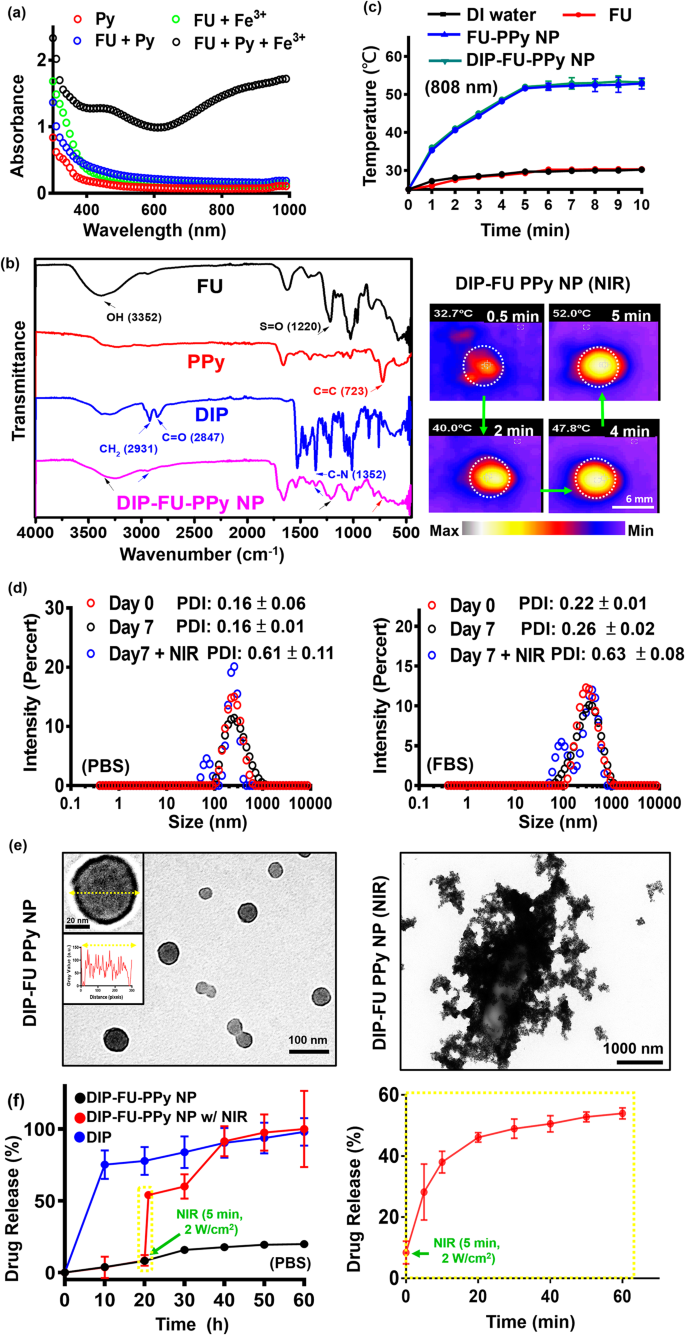
A UV-Vis spectroscopic technique [43] was used to substantiate the mechanism of PPy formation within the FU-PPy NP system. Determine 2a shows the UV-Vis spectroscopic information of the Py monomer (0.2 mg/mL, pink), answer (inexperienced) of FU (0.2 mg/mL) with ferric chloride (0.2 mg/mL), and answer (blue) of Py monomer (0.2 mg/mL) with FU (0.2 mg/mL). The UV-Vis spectroscopic information confirmed no apparent absorption at 600 − 800 nm (FU + ferric chloride, and FU + Py group) within the spectrum, suggesting the absence of Py monomer polymerization [43]. Aqueous options (black) containing the Py monomer (0.2 mg/mL) and FU (0.2 mg/mL) have been blended with ferric chloride (0.2 mg/mL), and we discovered a stronger depth at 600 − 800 nm. This might be attribute of the π − π* transition of PPy supplies with greater molecular weights (polymerization of Py monomers) [43]. These findings confirmed that ferric chloride was concerned within the oxidative polymerization of the Py monomer on this FU-PPy NP system. Comparable findings assist this oxidative technique for Py polymerization utilizing ferric chloride [31]. Within the case of NPs, it won’t be pure absorption, however extinction (absorption plus scattering), which is detected by UV-Vis spectroscopy. Fourier remodel IR (FTIR, Fig. 2b) spectroscopy supplied resolvable chemical structural data of check samples. The present FU polymer, together with its nano-construction, displayed attribute peaks, representing a hydroxyl group (OH, ca. 3352 cm− 1) with an S = O group (ca. 1220 cm− 1). PPy revealed peaks at C = C (ca. 723 cm− 1). DIP revealed its construction at CH2 (ca. 2931 cm− 1) and at C-N (ca. 1352 cm− 1). The resultant DIP-FU-PPy NPs indicated comparable positions as talked about (C–N at ca. 1352 cm− 1, N-H at ca. 3391 cm− 1, CH2 at ca. 2931 cm− 1, S = O at ca. 1220 cm− 1, C = C at ca. 723 cm− 1, and an OH purposeful group close to 3352 cm− 1). The FTIR and UV-Vis absorption spectroscopic analyses confirmed that DIP-FU-PPy NPs had been synthesized.
The photothermal impact of the examined nano-formulations after NIR irradiation confirmed that the PPy-based NPs produced hyperthermia in subsequent purposes (Fig. 2c). The temperature of the DIP-FU-PPy NP or FU-PPy NP group elevated upon NIR irradiation and reached a plateau after 5 min. The effectivity of photothermal conversion of DIP-FU-PPy NPs or FU-PPy NPs was ca. 25%, which was greater than that of FU or water alone. Gold-based NPs (AuNPs) have been investigated as photoablation brokers for most cancers remedy. Nevertheless, NIR-absorbing AuNPs have restricted photostability as a result of their absorption peaks and buildings might degrade after extended laser publicity [20]. Amongst NIR-absorbing nanomaterials, PPy-based supplies are notably essential as photoablation substances for regional PTT. Based on beforehand printed literature, NIR-conducting polymeric-based nanomaterials confirmed an identical charge of elevated temperature (ca. 10 °C/min) that produced biocompatible delicate hyperthermia for biomedical purposes [31].
To judge the soundness of the DIP-FU-PPy NPs in vitro, the NPs have been incubated with a PBS answer and PBS containing 10% serum (10% FBS) for 7 days at 37 °C. The PDI of DIP-FU-PPy NPs remained near 0.15 in PBS for 7 days, indicating that colloidal stability was maintained (Fig. 2d). The particle dimension and PDI of DIP-FU-PPy NPs barely elevated in these media, however the PDI was nonetheless lower than 0.27 as much as 7 days, indicating that no apparent aggregation had occurred (Fig. 2d). The TEM evaluation revealed modifications within the morphology and dimension of the NPs earlier than and after NIR irradiation (Fig. 2e). The NPs have been spherical with a core-shell construction previous to laser irradiation, and possessed an internal core PPy construction and an outer FU shell made of various elements. After laser irradiation, the particle dimension and PDI (as much as 0.6) considerably elevated, as revealed by the TEM and PDI analyses (Fig. 2d, e).
In vitro drug-release research
The drug-release habits of DIP was investigated by dialysis, each with and with out exterior stimuli. As proven in Fig. 2f, lower than roughly 10% of DIP was launched from the NPs within the dialysis buffer over a interval of 10 h (black). DIP was launched from DIP-FU-PPy NPs in a more-sustainable method in a PBS answer, and that launch was regular for as much as 20 h (roughly 10% decrease DIP was launched), suggesting that DIP-FU-PPy NPs might retard the DIP launch charge (Fig. 2f). The preliminary burst launch of DIP was thus decreased after being encapsulated in FU-PPy NPs. The impact of retardation could be attributed to steady interactions between DIP and the FU-PPy NPs, which ought to be just like electrostatic interactions between the anionic FU and cationic DIP. Moreover, PPy, with its hydrophobic domains, can simply bind to DIP. Thus, the DIP-FU-PPy NP advanced shaped was answerable for the decreased DIP launch charge. Nevertheless, the cumulative launch proportion of free DIP elevated quickly to ca. 70% after 10 h (blue).
The hyperthermic temperature generated by the embedded conductive PPy underneath NIR mild triggered the discharge of DIP (Fig. 2f, pink). The FTIR evaluation revealed that no structural modifications occurred between the hyperthermia-treated DIP group and the no hyperthermia-treated DIP group, indicating the chemical stability of DIP (Fig. 1S). After preliminary NIR irradiation, the cumulative launch proportion of DIP sharply elevated by ca. 4-fold in comparison with that within the non-NIR-treated DIP-FU-PPy NP group, representing a notably accelerated DIP launch course of (Fig. 2f). Upon irradiation with NIR mild, the NIR-photothermal impact of DIP-FU-PPy NPs triggered a morphological transformation of the polymeric provider and elevated the PDI worth to 0.6, as per DLS and TEM information (Fig. 2d, e). This allowed the therapeutic DIP to be launched from the NPs and have an effect on the thrombotic vessel. These information indicated that the constructed DIP-FU-PPy NPs with a core-shell construction might keep away from random leakage of the laden DIP into the blood circulation however then hasten drug launch on the lesion web site underneath NIR stimuli, thus producing most therapeutic efficacy and minimal unwanted side effects. Regardless of the promising purposes of this developed drug nanocarrier, its metabolism and potential toxicity stay to be absolutely elucidated, and additional investigations are wanted.
(a) UV-Vis (300 to 1000 nm) absorption spectroscopic evaluation of the formation of polypyrrole (PPy) utilizing ferric chloride. (b) Fourier remodel infrared (FTIR) outcomes exhibiting the chemical construction of the ready samples. (c) Outcomes of temperature modifications of the check liquid formulation after NIR remedy exhibiting quantitative evaluation with a thermocouple (TM-925, Lutron) till the temperature reached 25 °C, and a qualitative picture of those aqueous formulations with a thermal digital camera (A-BF, RX300, China) at 808 nm and a pair of.0 W/cm2 (scale bar: 6 mm). (d) Polydispersity index (PDI) and (e) TEM (scale bar: 100 nm) outcomes exhibiting morphological modifications of the spherical core-shell construction of the nano-formulation with colloidal stability (PBS or PBS plus 10% FBS at pH 7.4 for 7 days). After laser irradiation, the TEM picture reveals particle disruption and aggregation (scale bar: 1000 nm). (f) Spectrophotometric evaluation exhibiting the discharge of dipyridamole (DIP) from the nanoparticles (NPs) underneath PBS. Below irradiation with NIR mild, the photothermal impact of the DIP-fucoidan (FU)-PPy NPs triggered the morphological transformation of the polymeric provider, which allowed the DIP to be launched from the NPs
In vitro clot-lysis exercise
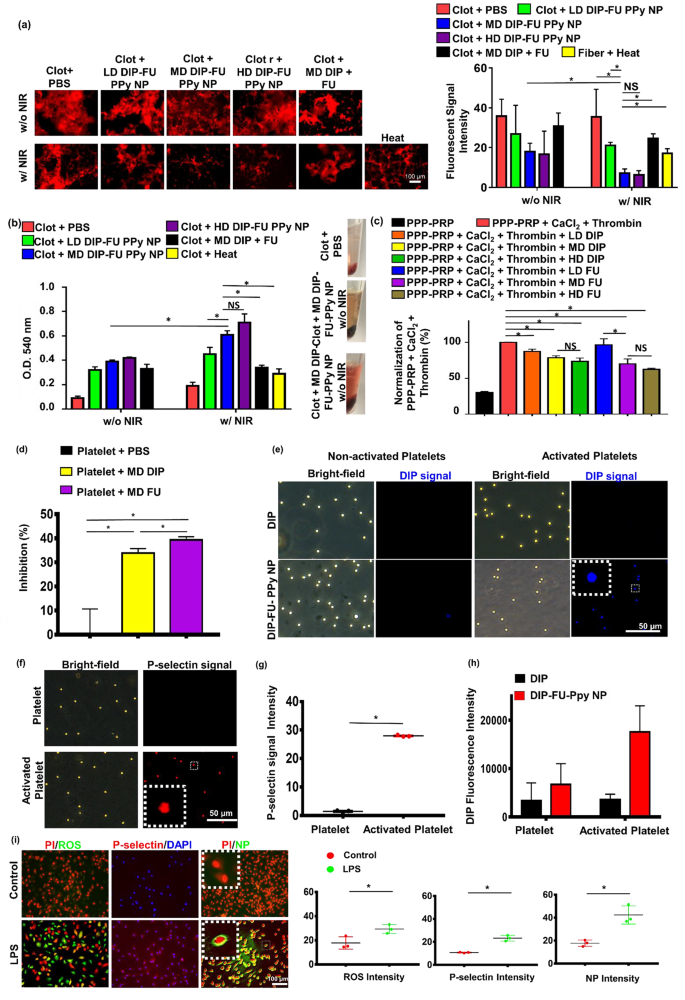
To confirm the bioactivity of the DIP-FU-PPy NPs at totally different doses (LD, MD, and HD), an in vitro blood clot–lysis evaluation was carried out utilizing fluorescent fibrin clot microscopic and Drabkin’s [23] strategies (Fig. 3a, b). With out 808-nm NIR (Fig. 3a), the LD, MD, and HD of DIP-FU-PPy NPs (0.1, 0.3 and 0.5 mg/mL) of the fluorescent fibrin clot have been respectively roughly 1.34-, 1.98-, and a pair of.15-fold decrease than these of the PBS group, indicating that the FU-decorated nano-formulation partially aided in vitro clot lysis. Upon irradiation with 808-nm NIR (Fig. 3a), clot absorbances of the LD, MD, and HD of DIP-FU-PPy NPs have been respectively roughly 1.68-, 4.99-, and 5.54-fold decrease than these of the PBS group, indicating that NIR irradiation-driven hyperthermia with NIR-triggered DIP launch additional facilitated in vitro clot lysis. These fluorescent information urged that NIR-driven hyperthermia strengthened the flexibility of the DIP-FU-PPy NPs to carry out in vitro thrombolysis. The thrombolytic exercise of MD DIP-FU-PPy NPs was considerably superior to that of the LD. Nonetheless, no important variations have been discovered between the MD and HD of DIP-FU-PPy NPs (Fig. 3a). The thrombolytic efficiency was additional examined utilizing Drabkin’s technique to colorimetrically assess the discharge of RBCs from the ready clots (Fig. 3b). Usually, when the extent of thrombolysis will increase, extra RBCs are launched from the clot, thereby rising the absorbance of the supernatant. Right here, the absorbance of the pink supernatant at 540 nm intensified because the extent of thrombolysis elevated; this improve was proportional to the colour change.
With out 808-nm NIR irradiation (Fig. 3b), the clot absorbances given for the LD, MD, and HD of DIP-FU-PPy NPs (0.1, 0.3, and 0.5 mg/mL) have been roughly 3.56-, 4.33-, and 4.67-fold higher, respectively, than these of the PBS group, indicating that FU ornament of the nano-formulation partially aided in vitro clot lysis. The clot + PBS management group confirmed a decrease absorbance at 540 nm, indicating fewer launched RBCs. Based on beforehand printed findings, FU possesses antiatherosclerotic and antithrombotic results [44, 45], which assist our outcomes. Upon irradiation with 808-nm NIR (Fig. 3b), the clot absorbances of the LD, MD, and HD of DIP-FU-PPy NPs have been roughly 2.37-, 3.21-, and three.74-fold higher, respectively, than these of the PBS group, indicating that NIR irradiation-driven hyperthermia with NIR-triggered DIP launch additional facilitated in vitro clot lysis. The clot + PBS plus NIR management group confirmed a decrease absorbance at 540 nm, indicating that fewer RBCs had been launched. These findings proved that localized NIR-driven hyperthermia augmented thrombolysis by DIP-FU-PPy NPs in vitro. The thrombolytic exercise of MD DIP-FU-PPy NPs was considerably greater than that of the LD. Nevertheless, no important variations have been noticed between the MD and HD of DIP-FU-PPy NPs (Fig. 3b). Due to this fact, the MD simulation of DIP-FU-PPy NPs was chosen for additional experiments.
A number of research demonstrated that DIP and FU exhibit antiplatelet properties and inhibit clot formation [46, 47]. In turbidimetric assays, we verified the era of fibrin clots by supplementing PPP-PRP with CaCl2 and thrombin. Subsequent, FU or DIP at numerous doses was supplemented with PPP-PRP along with agonists and CaCl2 to look at the corresponding results on inhibiting clot formation. As proven in Fig. 3c, FU and DIP decreased the speed of fibrin-clot formation, in line with the fibrin clot absorbance at 340 nm [48]. The decrease absorbance of the FU- and DIP-containing samples in a dose-dependent method signifies that FU and DIP had inhibitory results in opposition to the formation of fibrin clots. To inhibit platelet aggregation, FU or DIP was added to PPP-PRP along with agonists and CaCl2 to look at the corresponding results on the inhibition of platelet aggregation.
Platelet aggregation is a technique employed to guage platelet activation ranges and the extent of platelet-platelet binding in response to agonists [49]. Spectrophotometric evaluation is a typical method used to find out the aggregation exercise of platelets in vitro [50]. As proven in Fig. 3d, FU and DIP elevated the inhibition charge of platelet aggregation in line with the platelet aggregation absorbance at 595 nm [51]. A better (platelet aggregation) p.c inhibition of the FU- and DIP-containing samples than that of the PBS group was noticed, indicating that FU and DIP had inhibitory results in opposition to platelet aggregation. These outcomes are according to research reporting that inhibition of activated platelet aggregation and clot formation are bioactivities of FU and DIP [46, 47]. DIP was demonstrated to exert its antithrombotic results by means of numerous mechanisms. These embrace inhibiting platelet activation, affecting platelet operate, inhibiting the reuptake of adenosine by erythrocytes (which may result in vasodilation), influencing the expression of endothelial activation markers resembling von Willebrand issue antigen, and doubtlessly having anticoagulant results by lowering peak and complete thrombin era after a transient ischemic assault or ischemic stroke [52]. Based on beforehand printed findings, FU was proven to inhibit platelet activation by means of mechanisms resembling lowering the focus of endothelial microparticles and von Willebrand issue, in addition to inhibiting the movement of extracellular calcium ions [53].
A big improve in absorbance at 540 nm was noticed after the in vitro clots have been handled with the FU-decorated nano-formulation (MD DIP-FU-PPy NPs upon NIR irradiation; Fig. 3b), suggesting that the lytic efficiency considerably elevated owing to the NIR-photothermal responsive FU-decorated DIP nanocarrier. FU has excessive binding affinity towards P-selectin. Due to this fact, an enchancment within the therapeutic impact of DIP-FU-PPy NPs (Fig. 3) might even have been because of the elevated accumulation of therapeutics within the thrombus by focusing on P-selectin-overexpressing cells (activated platelets).
P-Selectin supply and activated platelet interactions
Binding capacities of DIP-FU-PPy NPs to non-activated and activated platelets have been assessed utilizing fluorescence microscopy. Non-activated platelets handled with DIP-FU-PPy NPs displayed weak DIP fluorescence indicators (blue). Nevertheless, the DIP depth significantly elevated in activated platelets in comparison with that in non-activated platelets handled with DIP-FU-PPy NPs or activated platelets handled with DIP. The fluorescence depth (verified utilizing ImageJ software program) of DIP-FU-PPy NPs in activated platelets was greater than these of DIP in non-activated and activated platelets, respectively (Fig. 3e-h). The fluorescence depth indicated that FU affected the particular binding of DIP-FU-PPy NPs to activated platelets, additional verifying the flexibility of Fu-decorated NPs to exactly ship therapeutics to the thrombus web site. Fluorescence microscopic information confirmed that the P-selectin degree in activated platelets had elevated 20-fold in comparison with that in non-activated platelets (Fig. 3f, g).
Along with activated platelets, LPS-treated endothelial cells exhibit irritation just like that seen in the course of the development of thrombosis [54] and induce intracellular P-selectin [55]. As proven in Fig. 3i, LPS-treated cells certainly yielded a major improve in intracellular inflammatory markers (ROS, roughly 1.65-fold stronger than within the management group) and P-selectin (roughly 2.17-fold stronger than within the management group), suggesting that infected endothelial cells had developed in vitro [56]. DIP [57] can inhibit blood clot formation when administered at greater doses over a brief interval owing to its hydrophobicity. Typical drug supply can’t trigger its particular accumulation in thrombotic areas, presumably leading to hostile unwanted side effects. Our developed nano-formulation, DIP-FU-PPy NPs, confirmed extremely focused accumulation towards infected endothelial cells (Fig. 3e, DIP sign) in comparison with that within the regular cell group (with out LPS) handled with DIP-FU-PPy NPs. The fluorescence depth of LPS-treated cells handled with DIP-FU-PPy NPs was roughly 2.38-fold greater than that of non-LPS-treated cells. FU allowed the DIP-FU-PPy NPs to connect to the plasma membrane of goal LPS-induced cells, in line with a magnified picture (Fig. 3i). Thus, this nano-development might obtain additional therapeutic efficacy in thrombotic situations.
Thrombolysis and in vitro clot-formation assay. Thrombolytic impact: supplementation with a low dose (LD), average dose (MD), and excessive dose (HD) of dipyridamole (DIP)-fucoidan (FU)-polypyrrole (PPy) nanoparticles (NPs) to in vitro clots. The quantitative thrombolytic exercise of formulations was evaluated utilizing (a) a microscopic assay (DFC7000T, LEICA) and (b) a colorimetric assay to evaluate the discharge of pink blood cells (RBCs) from blood clots through Drabkin’s technique. (c) Inhibition of in vitro clot formation utilizing colorimetric assays: the addition of LD, MD, and HD of DIP or FU to platelet-poor plasma (PPP)-platelet-rich plasma (PRP) (inhibition of in vitro clot formation) earlier than CaCl2 was added (ECHO CHEMICAL, 2.5 mM) and thrombin (Sigma-Aldrich, 1 U/mL). (d) Platelet aggregation utilizing colorimetric assays: the addition of the MD of DIP or FU to PPP-PRP within the presence of CaCl2 and thrombin. (e) Fluorescence microscopic (DFC7000T, LEICA) photos of non-activated and activated platelets (induced by TRAP-6, 10 µM) handled with MD DIP and DIP-FU-PPy NPs (scale bar: 50 μm). (f) Mobile P-selectin ranges assessed by microscope. Quantitative (g) P-selectin and (h) DIP ranges of non-activated platelets or activated platelets utilizing a microscopic assay. (i) Fluorescent microscopic information exhibiting a fluorescence picture of the nano-formulation interacting with or with out LPS-induced bovine aortic endothelial cells handled with 4’,6-giamidino-2-phenylindole (DAPI, Biotium #40,043, 1:1000, 30 min, 23 °C), propidium iodide (PI, ThermoFisher, 1.5 µM, 30 min, 23 °C), or 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, 40 µM, 30 min, 37 °C) for ROS, and NB100-65392 (1:500, 1 h, 37 °C) as a P-selectin marker (scale bar: 100 μm). Management group: cells with out LPS remedy. (* p < 0.05; NS, non-significant at p > 0.05)
In vivo thrombosis induction and in vivo vascular fluorescent evaluation
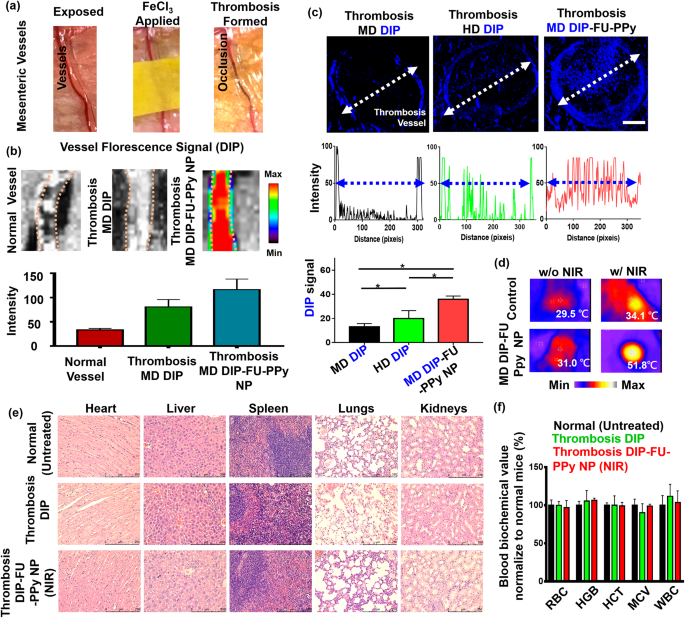
To raised perceive the improved efficacy of DIP-FU-PPy NPs within the ferric chloride-induced mouse mesenteric thrombus mannequin (Fig. 4a), thrombus focusing on and the penetration capability have been examined by IVIS and fluorescence microscopic imaging assays. As proven in Fig. 4b, after administration of DIP-FU-PPy NPs, a powerful DIP fluorescent sign was detected on the thrombus web site, indicative of its effectual thrombus focusing on due to selective binding to P-selectin across the floor of activated platelets on the thrombus web site. Nevertheless, solely weak fluorescent indicators have been detected for the vessel within the free DIP group with expression within the thrombus or the group in regular mice (Fig. 4b).
Determine 4c reveals that for the free DIP-treated group (MD, 3 mg/kg), very weak fluorescence was detected solely on the fringe of the thrombosis, suggesting a negligible affinity towards the thrombotic web site and inefficiency in penetrating the vessel construction and the thrombus heart. The HD of free DIP (5 mg/kg) demonstrated barely enhanced blue fluorescent indicators on the fringe of the thrombus, with a low sign inside its core (Fig. 4c). Remedy with DIP-FU-PPy NPs (MD, 3 mg/kg) precipitated a remarkably higher and deeper-blue fluorescent sign throughout the thrombus from the sting to the core in comparison with that of free-DIP (Fig. 4c). Based on earlier findings [58], a FU-functional supramolecular DDS was reported to favor deeper tissue penetration into vascular lesions, with the speculation that such nanoscale carriers possess elevated hemodynamic habits that to some extent, mimic the migration of leukocytes to vascular lesions. The deep penetration of the formulations might have been promoted by the nano-size of the ready carriers. As beforehand reported, a community of fibrin varieties pores smaller than 1 μm in thrombus clots [59], and the nanosized provider vary can penetrate deep into the inside of a clot. These nanocarriers can launch medication in response to NIR contained in the clot and facilitate intra-clot lysis, which is according to the upper thrombolytic exercise noticed under. This might be attributed to the efficient thrombus focusing on of DIP-FU-PPy NPs owing to the improved penetration of nanosized particles alongside the systemic circulation with selective binding by means of P-selectin interactions. These in flip facilitated the efficient penetration of therapeutics towards the thrombus web site and triggered the discharge of DIP therein.
In vivo efficacy, biodistribution, and biosafety research
Hyperthermia could be achieved as soon as the area of the thrombotic web site/vessel is irradiated with NIR, implying that exact and ample accumulation of NIR-photothermal switchable DIP-FU-PPy NPs occurred in vivo (Fig. 4d). The histological information (Determine S2) and beforehand printed findings [60,61,62] indicated {that a} NIR or delicate photothermal hyperthermic impact doesn’t trigger vessel damage. For in vivo security assessments, H&E staining revealed that DIP-FU-PPy-NP remedy, just like the opposite remedy teams (untreated wholesome management and free DIP), didn’t trigger irritation or notable toxicity in main tissues (liver, coronary heart, spleen, kidneys, or lungs, Fig. 4e). The hematological information proved that remedy with DIP-FU-PPy NPs (MD, 3 mg/kg) resulted in negligible modifications in blood indices (Fig. 4f). The key biochemical indices have been just like regular ranges in wholesome animals. These findings recommend that DIP-FU-PPy NPs are biologically secure in vivo. Quite a few investigations have supplied biocompatible NIR expertise at about 2 W/cm2 for precision remedy in a number of biomedical makes use of [63, 64]. Thus, using an NIR supply could be utilized in biomedicine.
As soon as the nanomedicine is run in vivo, a number of inherent immunological mechanisms start to determine and accumulate these international particles/substances and direct them towards the main elimination pathways from the physique. There’s thus all the time competitors between the specified distribution of nanomedicines in particular tissues and the extremely bioactive clearance mechanisms. The extent and distribution sample of a nanomedicine in several tissues and organs, throughout a scientific therapeutic or diagnostic software, are often thought of the biodistribution. The speed of their recognition and removing by the immune system, excretion, and metabolism from the physique is usually known as pharmacokinetics. Figuring out the biodistribution and pharmacokinetic traits is significant to augmenting the anticipated performance of a nanomedicine in any chosen area or tissue of the physique and minimizing toxicological unwanted side effects due to any undesirable pharmacokinetic behaviors or biodistributions [65]. To the perfect of our data, research carried out to analyze PPy-based nanocarrier metabolic and biodistribution habits are uncommon.
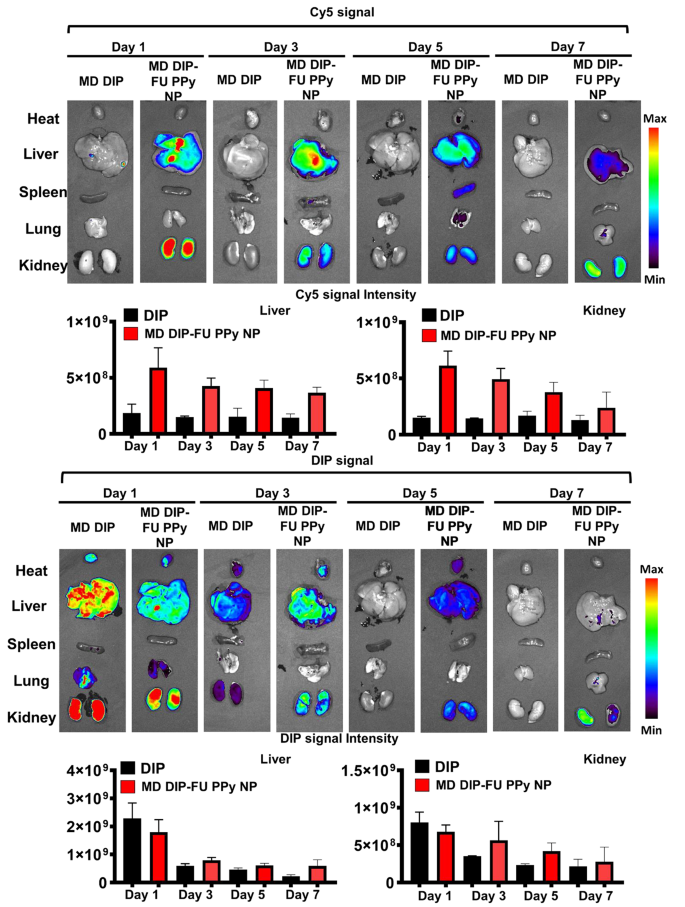
To check the metabolic impact behaviors and biodistribution of DIP-FU-PPy NPs, check mice have been administered free DIP or DIP-FU-PPy NPs. As proven in Fig. 5, free DIP was shortly eradicated from physique at 1 day, and most DIP indicators have been discovered within the liver and kidneys. Quite the opposite, comparatively low indicators within the liver and kidneys have been present in DIP-FU-PPy NP-treated mice. The extended circulation was attributed to the small particle dimension, colloidal stability, and adverse cost of DIP-FU-PPy NPs. It appeared that DIP-FU-PPy NPs exhibited important retention capabilities.
DIP-FU-PPy NPs confirmed an absolute benefit over free DIP within the battle of thrombus focusing on, avoidance of clearance, and retention results, which was evidenced by fluorescence photos. Extra importantly, the fluorescence of DIP and the Cy5 provider in excised liver and kidneys lasted so long as 7 days and was step by step eradicated by means of the kidneys (Fig. 5). The upper drug focusing on, retention, eventual degradation, and elimination skills might lead to higher biocompatible, therapeutic, and metabolic responses. These findings urged that DIP-FU-PPy NPs confirmed good nanosystem circulation, biosafety, and enhanced bioavailability, retention, and metabolic skills.
(a) Photographic observations demonstrating the creation of a thrombosis mannequin utilizing a ferric chloride filter paper technique (filter paper was soaked in a 35% FeCl3-6H2O answer and positioned on the recognized vessel). (b) IVIS (IVIS Lumina III XRMS) information exhibiting a qualitative picture and qualitative evaluation of fluorescent dipyridamole (DIP) ranges alongside the dotted traces of various formulations (average dose (MD) DIP or MD DIP-fucoidan (FU)-polypyrrole (PPy) nanoparticles (NPs)). (c) Fluorescence depth of DIP in clot in fluorescence photos of thrombus websites as analyzed by ImageJ software program. The biodistribution outcomes indicated that thrombi handled with free-DIP have been unable to explicitly accumulate DIP or enable it to penetrate on the thrombus web site. The thrombus web site of the group given DIP-FU-PPy NPs exhibited deeper penetration and higher accumulation of DIP. (d) Thermal imaging (A-BF, RX300) exhibiting that domestically utilized NIR (808 nm, 2.0 W/cm2 for 10 min) might induce hyperthermia within the group that obtained DIP-FU PPy NPs. (e) Consultant photomicrographs of H&E-stained smooth tissues (liver, coronary heart, lungs, spleen, and kidneys) after remedy with DIP-FU-PPy NPs. H&E-stained microscopic and hematological information indicated that remedy with DIP-FU-PPy NP had in vivo organic security (scale bar: 250 μm). (f) Hematological research and biochemical indexes (pink blood cells (RBCs), hemoglobin (HGB), hematocrit (HCT), imply corpuscular quantity (MCV), and white blood cells (WBCs)) assessed utilizing a blood serum hematological analyzer (Procyte Dx, IDEXX Laboratories). Experimental outcomes are introduced because the imply ± SD (n = 6). * p < 0.05; NS (non-significant) at p > 0.05). (Average dose (MD) DIP equal: 3 mg/kg physique weight; excessive dose (HD) DIP equal: 5 mg/kg physique weight)
In vivo imaging and biodistribution examine. Actual-time in vivo fluorescence photos of free-form dipyridamole (DIP) and DIP-fucoidan (FU)-polypyrrole (PPy) nanoparticles (NPs) conjugated with Cy5 in check mice at 1, 3, 5 and seven days, assessed by IVIS. Semiquantitative fluorescence outcomes of kidney and liver tissues. The consultant organs in every group of IVIS photos (for DIP or Cy5 distribution) could also be both the identical or totally different in vivo
In vivo evaluation on the effectiveness of P-selectin focusing on/inhibition, its antithrombotic efficacy, and doable results
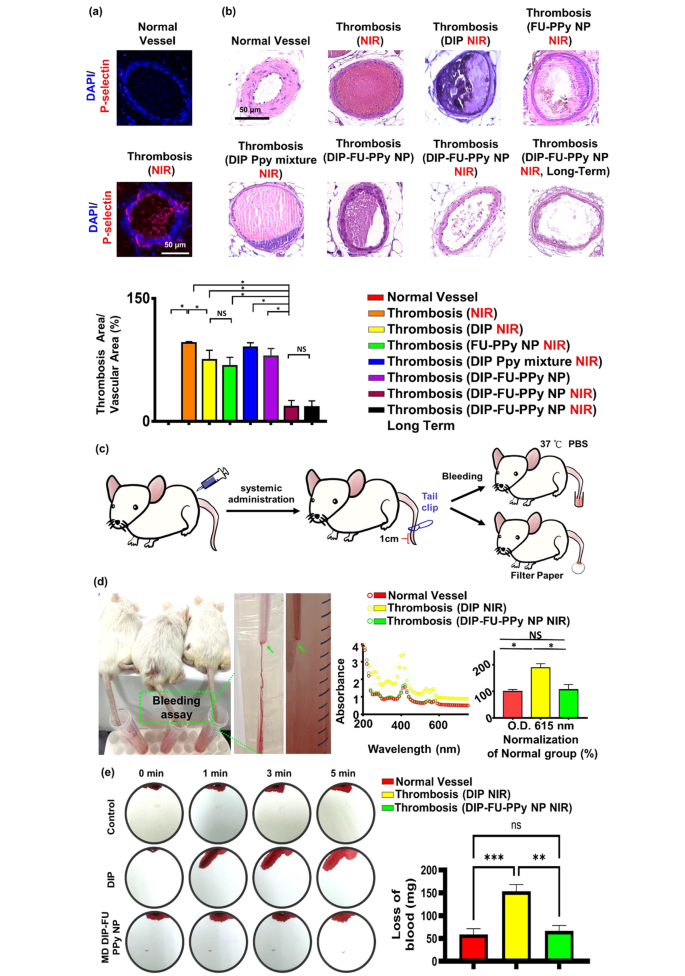
P-Selectin is expressed on the floor of activated platelets and is a thromboinflammatory biomolecule engaged in platelet aggregation and activation. As well as, FU has a excessive affinity for P-selectin and is, subsequently, appropriate for particular therapeutic supply and diagnoses of bioactivities related to P-selectin expression, resembling pathological thrombotic organs. Knowledge obtained from a microscopic analysis of vessel thrombosis after ferric chloride publicity are proven in Fig. 6a. Publicity to ferric chloride precipitated giant, compact thrombi, which carefully resembled the thrombus phenotype. Ferric chloride-induced thrombus formation and elevated in vivo P-selectin expression are identified to be related [66]. An immunofluorescent histological evaluation revealed that P-selectin was extremely expressed in thrombotic vascular clots (greater than that within the management group; Fig. 6a). The FU nanocarrier particularly collected in thrombotic clots. The vessel floor on the ferric-treated vascular thrombosis web site expressed P-selectin, resulting in fibrin clot formation, as noticed in our histological information (Fig. 6a).
As proven in Fig. 6b, a plugged thrombus was clearly retained within the management group (thrombosis + NIR). Remedy with free DIP (MD, 3 mg/kg BW) resulted in a slight lower within the thrombus dimension. It was discovered that the retained thrombus after the free-DIP remedy underneath NIR irradiation had a clean floor, brought on by poor penetration (Fig. 6b), and the resultant antithrombotic bioactivity was restricted to the floor of the thrombus. In distinction, remedy with DIP-FU-PPy NPs (MD, 3 mg/kg BW) with NIR irradiation led to important thrombus dissolution (with solely round 17% of the clot space remaining), additional confirming that the antithrombotic efficacy was considerably greater than that of the management (with round 95.73% of the clot space remaining) and free-DIP teams (with round 75.03% of the clot space remaining). Histological information from check animals administered DIP alone with subsequent NIR remedy confirmed an anticlotting impact on the induced thrombus web site. This discovering suggests {that a} appreciable dose of DIP is required to realize an anticlotting impact. Check animals administered the blended formulation of DIP and PPy with out FU however with NIR irradiation confirmed negligible thrombus dissolution (with round 90% of the clot space remaining). Check animals administered DIP-FU-PPy NPs with out NIR irradiation revealed a restricted thrombus dissolution efficacy (with round 79.18% of the clot space remaining, Fig. 6b). Check animals administered FU-PPy NPs with NIR irradiation revealed a partial thrombus dissolution end result (with 67% of the clot space remaining). These findings urged that hyperthermia, FU and DIP have been concerned within the antithrombotic motion. Due to this fact, animals administered FU-based NPs with subsequent NIR remedy exhibited appreciable reductions in clots within the vascular lumen in comparison with thrombotic animals administered systemic DIP or thrombosis alone with subsequent NIR. This suggests that the administered DIP-FU-PPy-NPs particularly collected on the FeCl3-induced P-selectin-expressing thrombotic web site to facilitate the anticlotting efficacy of the mixed remedy (DIP and photothermal ablation) (Fig. 6b).
Histological and quantitative outcomes confirmed that after animals have been administered DIP-FU-PPy-NPs and subjected to sequential irradiation with NIR, regeneration of the thrombosis was prevented for 1 day (Fig. 6b). The superior antithrombotic effectivity and prevention of thrombus regeneration have been attributed to augmentation of the antithrombotic effectiveness of the NIR-triggered launch of DIP and hyperthermia. The incorporation of FU-decorated P-selectin-targeting NPs exactly delivered these therapeutics to the thrombus clot.
A rodent tail-bleeding assay was used to evaluate the chance of hemorrhaging with DIP-FU-PPy-NPs (Fig. 6c, d). On this assay, a higher tail-bleeding quantity was related to the next hemorrhage threat [67]. As proven in Fig. 6d, the distal 1-cm phase of the tail of a mouse was excised utilizing a medical scalpel to induce bleeding, and the extent of bleeding was decided at 1 h after administration of free-DIP (NIR) or DIP-FU-PPy-NPs (NIR) (MD, 3 mg/kg BW). The bleeding amount induced with free DIP (MD, 3 mg/kg BW) remarkably elevated in comparison with that induced by DIP-FU-PPy-NPs. As proven in Fig. 6d, the bleeding extent assessed by the hemoglobin absorbance assay in animals handled with free DIP was considerably higher than that within the DIP-FU-PPy-NP-treated group (because the absorbance elevated round 1.78-fold relative to that within the DIP-FU-PPy-NP-treated group). A comparability between the traditional animals and thrombotic animals handled with DIP-FU-PPy NPs and NIR yielded no statistically important variations within the bleeding assay outcomes (Fig. 6d). This means that FU-modified NPs localized the therapeutics towards the thrombotic area after which initiated exact supply of DIP upon NIR remedy to provoke site-specific therapeutic/photothermal thrombolytic results with nice therapeutic effectivity. Thus, hostile occasions on account of therapeutic-generated hemorrhaging in regular organs have been averted. The extent of bleeding from the liver as assessed utilizing blood absorption onto filter paper in animals handled with free DIP (NIR) was considerably higher than that within the DIP-FU-PPy-NP-treated group (blood absorption onto filter paper elevated about 2.3-fold relative to the DIP-FU-PPy-NP-treated group) (Fig. 6e). These findings indicated that DIP-FU-PPy-NPs can remarkably scale back nonspecific hemorrhagic unwanted side effects of free DIP used for thrombus remedy. As beforehand reported, the scientific use of DIP in thrombotic remedy will increase the chance of extreme bleeding [68]. Huge hemorrhaging attributed to the uncontrolled launch of thrombolytic medication is a vital concern in thrombus remedy in scientific apply [69]. The enrichment supplied by DIP-FU-PPy-NPs resulted in augmented DIP supply, elevated thrombolytic efficacy, and a decreased threat of hemorrhaging.
(a) In vivo histological outcomes of fluorescent microscopy exhibiting the expression of P-selectin in vessels. (b) In vivo outcomes of sunshine microscopy exhibiting vascular H&E histological modifications (scale bar: 50 μm), thrombus remedy, and prevention of thrombus regeneration in animals handled with totally different formulations. The antithrombotic efficacy was analyzed utilizing the next technique: (histologically microscopic vascular clot space/lumen space) × 100%. The calculated areas or microscopic fluorescence intensities have been analyzed utilizing ImageJ software program. (c) Schematic illustration depicting the tail bleeding check. (d) For the tail bleeding check, after administration and remedy with dipyridamole (DIP)-fucoidan (FU)-polypyrrole (PPy) nanoparticles (NPs) or the identical quantity of DIP (average dose (MD), 3 mg/kg physique weight), the distal 1-cm part of a mouse tail was lower off utilizing a scalpel and submerged in PBS prewarmed to 37 °C. Animals have been noticed for 30 min. To calculate the bleeding amount, the bleeding extent was assessed primarily based on the hemoglobin degree within the PBS answer by figuring out the absorbance spectra of hemoglobin. (e) For an additional bleeding check, filter paper was used to soak up blood from the bleeding liver of check animals. Blood-diffused areas on the filter paper have been measured utilizing ImageJ software program. Microscopic and biochemical information confirmed that the DIP-FU-PPy NPs possessed antithrombotic efficacy and averted clot reformation and bleeding threat in vivo. Experimental outcomes are introduced because the imply ± SD (n = 6). (* p < 0.05; NS (non-significant) p > 0.05)
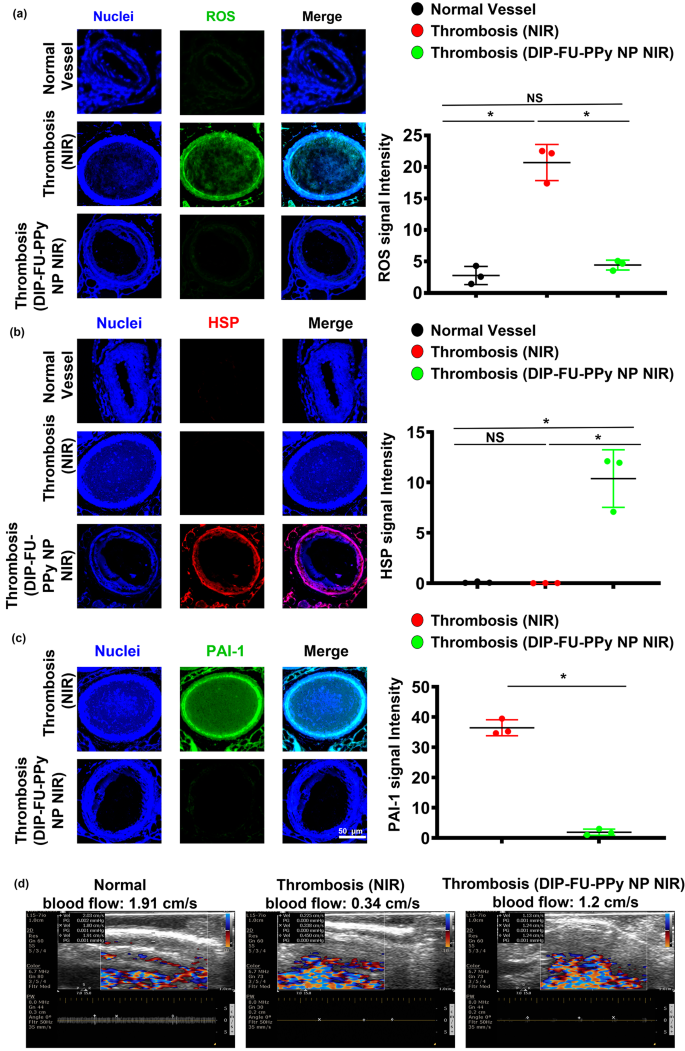
Oxidative stress is an important occasion underneath each pathological and physiological conditions. As no single genetic biomarker or check exactly predicts the extent of vascular well being, phenotyping for biomarkers of irritation ought to determine people in danger for vascular sicknesses. Reactive oxygen species (ROS) are essential mediators of signaling pathways that trigger vascular irritation in thromboses [70]. On this examine, we quantified oxidative stress by measuring mobile ROS utilizing DCFH-DA staining of vascular tissues. Microscopic information confirmed that thrombotic vessels had the best ROS ranges, in comparison with regular vessels of wholesome animals (Fig. 7a). Varied animal thrombosis fashions assist the notion that enhanced vascular ROS manufacturing from a dysfunctional mitochondrial respiratory chain performs a causative position in thromboses and different vascular illnesses [70]. Anti-inflammatory approaches can forestall thrombotic occasions [71]. The vascular ROS degree of thrombotic animals that obtained DIP-FU-PPy NPs plus NIR after long-term remedy confirmed comparable outcomes as regular vessels. The microscopic information indicated that the vascular HSP expression degree of thrombotic animals that obtained DIP-FU-PPy NPs plus NIR after long-term remedy was greater than these of the untreated thrombus and regular management teams (Fig. 7b). Based on beforehand printed findings, hyperthermia-induced HSP prevents or arrests inflammatory injury [72]. Together with the delivered anticoagulant, DIP, the phototherapeutic DIP-FU-PPy NPs produced a vascular anti-inflammatory impact plus HSP expression which was concerned with restoration of vascular injury and avoidance of rethrombosis. Microscopic information indicated that the expression degree of vascular plasminogen activator inhibitor (PAI)-1 of thrombotic animals that obtained DIP-FU-PPy NPs plus NIR after remedy was decrease than that of the untreated thrombus group (Fig. 7c). We verified vascular blood movement velocities with totally different remedies (Fig. 7d). The vascular blood movement velocity was measured utilizing ultrasound. In comparison with the traditional management group, the vascular blood movement velocity on the thrombotic web site was decreased, whereas the vascular blood movement velocity within the DIP-FU-PPy NPs plus NIR remedy group was mainly unchanged. These findings revealed that the fibrin clot was lysed on account of dissolution of the thrombus after DIP-FU-PPy NPs plus NIR remedy, and the vascular blood movement velocity had returned to regular.
In earlier research [61, 73], we developed PPy-based provider techniques utilizing the NIR-photothermal impact for thrombus remedy. A fibrin-targeting peptide-engineered nanoassembly of photothermal brokers and the antiplatelet, ticagrelor, was ready for thrombus-targeting supply with a excessive thrombolysis efficacy [60]. Nevertheless, the dearth of the flexibility to keep away from clot recurrence, bleeding threat, and data concerning the underlying mechanisms of the inhibitory impact of rethrombosis, restoration of broken vessels, biodistribution, and provider degradability restricted its efficiency in thrombus administration. On this examine, we designed DIP-FU-PPy-NPs that possessed thrombus-homing capabilities and NIR-phototherapeutic properties (discuss with Figs. 1, 2 and 3). DIP-FU-PPy NPs demonstrated an amplified thrombolysis efficacy, and the flexibility to forestall clot recurrence, reduce the bleeding threat, and inhibit rethrombosis by means of well-understood mechanisms (discuss with Figs. 4, 5, 6 and 7). Moreover, the DIP-FU-PPy NPs promoted restoration of broken vessels, exhibited favorable biodistribution, and demonstrated provider degradability.
Exact drugs necessitates correct early diagnoses and distant noninvasive and individualized correct remedies, and phototheranostics are thought of to be the frontier for offering secure and fast illness localization and efficient therapies. Semiconducting nanomaterials, resembling organically conjugated polymers, small molecules, and inorganic semiconductors with photonic options, are being explored in medical imaging and phototherapy (photodynamic, photothermal, and photo-controlled mixture therapies). In sensible scientific purposes, semiconducting natural supplies are favored over inorganic supplies for phototheranostics owing to their pure metabolism and biocompatibility. The supramolecular self-assembly method is considered a major technique of getting ready multifunctional and natural removable phototheranostics with supramolecular interactions, resembling hydrophobic results, electrostatic and π–π interactions, and hydrogen bonding, that are dynamic and noncovalent.
In scientific expertise, laser phototherapy was confirmed to be secure, efficient, and legitimate for thrombolysis [74,75,76,77]. Biocompatible NIR expertise takes benefit of the low absorption spectrum of organic tissues within the NIR vary (650–950 nm) and may penetrate deep tissues. Research confirmed that NIR performs an important position notably in scientific purposes the place deep tissue organ penetration (for tendons, bones, ligaments and cartilages) is required [78]. In scientific apply, NIR laser ablation has been effectively reported as a minimally invasive technique to deal with thrombotic illnesses, resembling varices of the decrease extremities [79]. On account of sturdy optical absorption by thrombi, lasers can be utilized to dissolve clots and, subsequently, can function an rising possibility in sufferers with sophisticated thromboses who fail to reply to thrombolytic medication and exhibit thrombotic lesions which can be deemed unsuitable for traditional balloon angioplasty [75]. Focused nano-DDSs have proven nice potential as clinically translatable phototheranostic brokers for treating thromboembolisms. General, there’s a sturdy prerequisite for additional enhancements and growth of photo-thrombolysis, resulting in its scientific translation [18]. As to future prospects, using NIR mixed with minimally invasive angiographic applied sciences can notice phototherapy in deep vascular lesions [80].
In vivo therapeutic mechanism. Microscopic information and semiquantitative fluorescence outcomes of vascular (a) reactive oxygen species (ROS), (b) anti-heat shock protein (HSP), and (c) anti-plasminogen activator inhibitor (PAI)-1 ranges of check mice (regular, thrombotic animals that obtained close to infrared (NIR) irradiation, and thrombotic animals that obtained dipyridamole (DIP)-fucoidan (FU)-polypyrrole (PPy) plus NIR). (d) Ultrasonic information of vascular blood movement velocities of check mice (regular, thrombotic animals that obtained NIR, and thrombotic animals that obtained DIP-FU-PPy plus NIR). * p < 0.05; NS (non-significant) at p > 0.05)