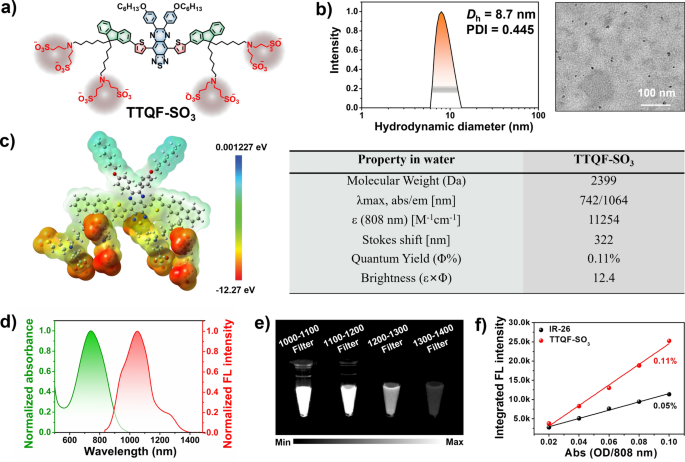
The chemical construction of TTQF-SO3 is proven in Fig. 1a, and its detailed artificial route is displayed in Further file 1: Scheme S1. First, the D–A–D-based NIR-II dye TTQF-NH2 with useful amino teams was achieved from our beforehand reported methodology. Subsequently, TTQF-SO3 was obtained after sulfonation of the intermediate TTQF-NH2 with extra 1,3-propanesultone. The goal TTQF-SO3 compound was completely characterised by NMR spectroscopy and MALDI-TOF–MS evaluation (Further file 1: Figs. S1, S2). The eight terminal charged facet chains of TTQF-SO3 allow a solubility in aqueous media better than 100 mg mL−1, such that TTQF-SO3 dissolves into water immediately with out the help of any natural solvents (like dimethyl sulfoxide or alcohols) or encapsulation poly(ethylene glycol) coatings). Dynamic gentle scattering and transmission electron microscopy outcomes revealed that TTQF-SO3 can self-assemble to type ultra-small dots with 8.7 nm common dimension (Fig. 1b). Zeta potential measurement revealed a unfavorable floor cost (− 14.3 mV) for TTQF-SO3 in PBS (Further file 1: Fig. S3a). Moreover, TTQF-SO3 saved a great colloidal stability both in phosphate-buffered saline (PBS) and dulbecco’s modified eagle medium (DMEM) inside 14 days (Further file 1: Fig. S3b). Another key properties of TTQF-SO3 in water media is proven in Fig. 1c. The absorption and fluorescence emission spectra of TTQF-SO3 have been measured. The attribute absorption and emission peaks have been noticed at round 742 nm and 1064 nm, respectively, with a major stokes shift of 322 nm (Fig. 1d). The molar extinction coefficient of TTQF-SO3 at 808 nm was decided to be 11,254 M−1 cm−1 (Further file 1: Fig. S4). The NIR-II alerts of TTQF-SO3 have been in contrast utilizing numerous long-pass (LP) filters (1000–1400 nm) on the identical focus in water media, and located that the fluorescence alerts might be clearly noticed with every filter, even with the 1300–1400 nm LP filter (Fig. 1e). The quantum yield (QY) of TTQF-SO3 was decided to be 0.11%, utilizing IR26 as a reference (QY = 0.05% in DCE, Fig. 1f and Further file 1: Fig. S5). Furthermore, the photostability of the TTQF-SO3 was a lot superior to that of IR1061, below steady laser irradiation (Further file 1: Fig. S6). All these outcomes might be adequate to assist TTQF-SO3 for NIR-II medical imaging prospects.
a Chemical construction of TTQF-SO3. b DLS and TEM picture of TTQF-SO3 in water. c Physicochemical properties of TTQF-SO3. d Normalized absorption and emission spectrum of TTQF-SO3 in water. e NIR-II alerts of TTQF-SO3 obtained with totally different filters on the identical focus (0.1 mg mL−1) in water. f The built-in fluorescence spectra of TTQF-SO3 and its versus totally different absorbance at 808 nm (IR-26 as reference, QY = 0.05% in dichloroethane)
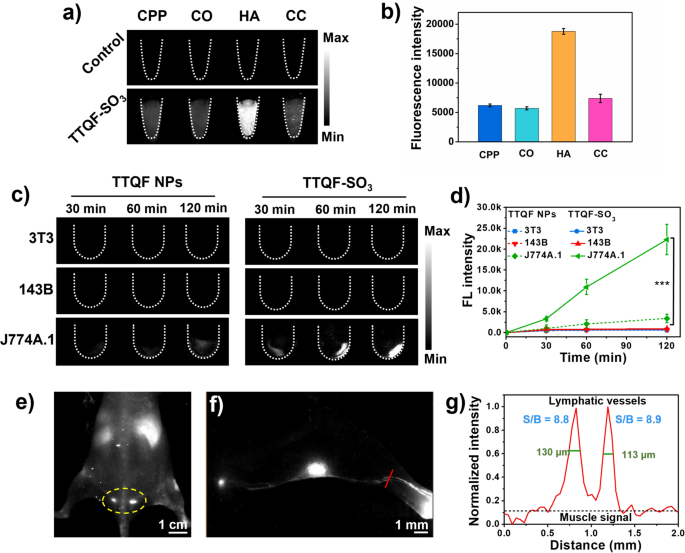
The affinity binding potential to bone tissue minerals is often an vital criterion for evaluating bone-targeted imaging. To confirm whether or not in vitro binding affinity of TTQF-SO3 towards inorganic calcium salt, totally different calcium-binding exams have been investigated, together with calcium pyrophosphate (CPP), calcium oxalate (CO), hydroxyapatite (HA), and calcium carbonate (CC). As proven in Fig. 2a, TTQF-SO3 demonstrated sturdy affinity for HA in comparison with different calcium salts, and its fluorescence depth was about 3-folds than that of different calcium salts (Fig. 2b), which might be attributed to the hydroxyl group of HA contributes to type a robust hydrogen bond with the sulfonyl anion of the fluorophore. [21, 25] Since HA is a significant element of bone, we anticipated that TTQF-SO3 would have a great bone-targeted potential in vivo.
a Calcium-binding NIR-II fluorescence picture (NIR-II FI) of TTQF-SO3. Imaging parameters: 1300 filter, publicity 500 ms. b Corresponding quantitative NIR-II fluorescent alerts of TTQF-SO3. c NIR-II FI of various cells (3T3, 143B, and J774A.1) handled with TTQF NPs and TTQF-SO3 at totally different time factors. Imaging parameters: 1300 filter, publicity 3000 ms. d Corresponding quantitative NIR-II fluorescent alerts of TTQF NPs and TTQF-SO3 sure cells. e NIR-II FI of TTQF-SO3 in mice after intravenous injection. Imaging parameters: 1300 filter, publicity 3000 ms. f NIR-II FI of lymphatic vessels after footpad injection of TTQF-SO3 at 60 min. g Cross-sectional sign profile of lymphatic vessels within the chosen area. Error bars, imply ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
Motivated by the above promising outcomes, we carried out additional exams on the mobile degree. As is well-known, the interplay between nanomaterials and cells primarily consists of electrostatic interplay, water transport interplay, hydrogen bond interplay, π–π stacking and steric hindrance [26]. Amongst them, the floor cost properties of nanoparticles not solely have an amazing impact on blood circulation, but in addition on cell uptake [27]. Due to this fact, three cell strains related to bone metabolism, together with embryonic fibroblast choice (3T3), osteosarcoma (143B), and mouse mononuclear macrophages (J774A.1), have been chosen for cell uptake research [28]. The non-functionalized precursor TTQF nanoparticles (NPs) was ready as a management group by co-deposition with the amphiphilic Pluronic F-127. Detailed traits of TTQF NPs have been listed in Further file 1: Fig. S7. The three sorts of cells have been incubated with TTQF-SO3 and TTQF NPs, respectively for 30, 60, and 120 min, and their fluorescence alerts have been monitored by NIR-II imager. As proven in Fig. 2c, there was a major distinction within the fluorescence depth of the 2 probes with macrophages at 120 min (P < 0.001). In distinction, the uptake of the 2 probes in 3T3 and 143B cells was almost no distinction. Extra precisely, these two probes should not simple to be absorbed by 3T3 and 143B cells in a short while. Contemplating that the QY (0.24%) of TTQF NPs is 2.2-fold than that of TTQF-SO3 (0.11%), TTQF-SO3 exhibited 14.7-fold increased uptake in J774A.1 cells than that of TTQF NPs at 120 min (Fig. 2d, Further file 1: Fig. S5). This outcome indicated that TTQF-SO3 was simply absorbed by undifferentiated monocytes, which can be carefully associated to its floor cost.
Given the superb macrophage uptake conduct of TTQF-SO3 in vitro, we additional studied its organic distribution in vivo. As illustrated in Further file 1: Fig. S8a, with 30 min after the injection of TTQF-SO3 (1.0 mg mL−1, 100 μL) by the tail vein, the vascular sign decreased considerably, whereas the liver sign elevated remarkably, indicating that the probe was quickly captured by the endothelial reticular system [29]. It’s value noting that the mouse tail lymph might be clearly noticed on the time level of two h, indicating that TTQF-SO3 can enter the lymphatic system by blood circulation (Fig. 2e). This outcome might be attributed to its affordable unfavorable cost density, which allows it to cross by the interstitial matrix full of collagen fibers and negatively charged glycosaminoglycans [30]. Moreover, the lymph nodes and lymph vessel (LV) might be clearly noticed after footpad injection of TTQF-SO3 at 60 min (Fig. 2f; Further file 1: Fig. S8b). The results of cross-sectional sign profile of LVs displayed the LVs-to-muscle ratios of ∼ 8.9 with remarkably sharp options (the complete width at half-maximums (FWHMs) of LVs was ∼ 130 μm). General, TTQF-SO3 might present different modalities for imaging the lymphatic system intraoperatively.
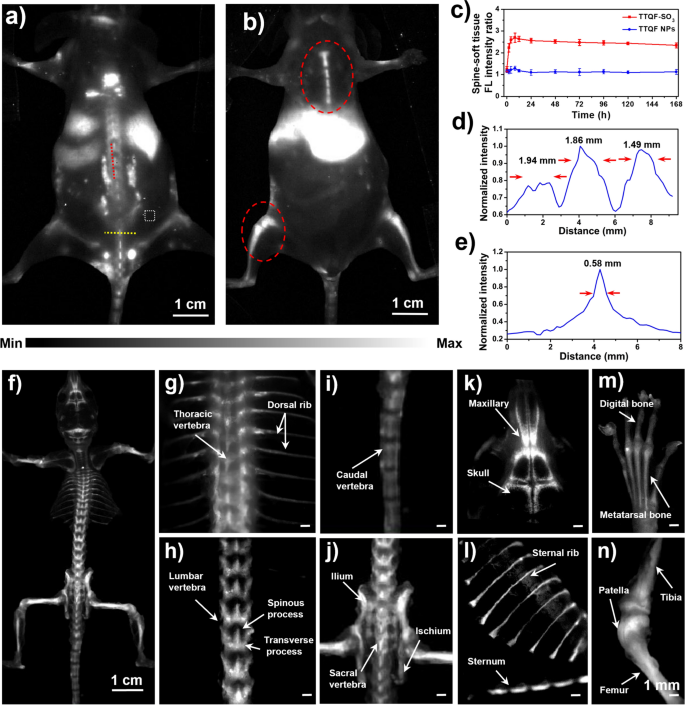
Earlier than analysis of bone-targeted imaging, the cytotoxicity of TTQF-SO3 was investigated. As demonstrated in Further file 1: Fig. S9a, 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays confirmed that TTQF-SO3 confirmed very low cytotoxicity, and the survival charge of cells might attain greater than 90% even at excessive focus (0.2 mg mL−1). Hemolysis additionally confirmed that TTQF-SO3 had very low biotoxicity, even at 4 mg mL−1 focus (Further file 1: Fig. S9b). Subsequent, TTQF-SO3 was intravenously injected (1.0 mg mL−1, 100 μL) into the tail of regular mice to measured its biodistribution utilizing the 1300 nm LP filter. As well as, the non-functionalized precursor TTQF NPs was ready as a management group by co-deposition with the amphiphilic Pluronic F-127. From NIR-II FI outcomes (Fig. 3a, b; Further file 1: Figs. S10 and S12), the TTQF-SO3 supplied a high-sensitivity and signal-to-background ratios (SBRs) within the regular bones in contrast with TTQF NPs (Further file 1: Figs. S11 and S13), revealing that the bones have been simpler to be acknowledged by a sulfonated fluorophore (TTQF-SO3) slightly than an unsulfonated fluorophore (TTQF). The SBR of the backbone was reached a peak of two.7 at 8 h after injection with TTQF-SO3 (Fig. 3c). Against this, the SBR was solely reached a peak of 1.2 at 8 h after injection with TTQF NPs, which was far under than that of TTQF-SO3 at virtually all time factors (Fig. 3c). Moreover, the above NIR-II FI outcomes have been quantitatively analyzed. As noticed in Fig. 3d, parallel evaluation (crimson line) alongside the backbone confirmed three peaks of fluorescence depth within the mouse injected with TTQF-SO3, excessive sufficient to differentiate totally different vertebrae. The FWHMs have been starting from 1.49 to 1.94 mm. Vertical evaluation crossing the backbone (yellow line) additionally exhibited a great differentiation (FWHM = 0.58 mm) between the vertebrae and others (Fig. 3e).
a, b NIR-II FI of BALB/c mice at 12 h post-injection utilizing TTQF-SO3. White dotted field: smooth tissue. Imaging parameters: 1300 filter, publicity 3000 ms. c Backbone-soft tissue fluorescence ratio of TTQF-SO3 and TTQF NPs at totally different time-point. d FL depth evaluation of the crimson line in a. e FL depth evaluation of the yellow line in a. f NIR-II FI of the entire skeletal system utilizing TTQF-SO3. g–n Stereomicroscope NIR-II FI of some pivotal skeletal system utilizing TTQF-SO3. Imaging parameters: 1300 filter, publicity 500 ms. Error bars, imply ± SD (n = 3)
Then, the ex vivo NIR-II FI was carried out on remoted bone tissues of the wholesome mouse after injected with TTQF-SO3. As displayed in Fig. 3f, the complete skeletal system might be clearly imaged from the susceptible place after eradicating smooth tissues. Furthermore, the small print of bone photographs at numerous websites have been additionally carried out utilizing a NIR-II stereomicroscope (Fig. 3g–n). Determine 3g clearly shows the thoracic vertebrae and dorsal rib of the conventional mouse. Determine 3h clearly displays the lumbar. It’s value noting that the spinous and transverse processes might be noticed within the photographs. Equally, the caudal vertebrae can be noticed in Fig. 3i. As well as, Fig. 3j exhibits the ilium and ischium, and Fig. 3okay shows the cranium of the mouse. Likewise, the next photographs displays the sternum and sternal rib (Fig. 3l), digital and metatarsal bone (Fig. 3m), tibia, patella, and femur (Fig. 3n) in flip. It must be famous that the smooth ribs of the chest weren’t proven in Fig. 3l, which implies that TTQF-SO3 couldn’t be enriched within the cartilage tissue on account of various composition of cartilage and bone tissue [31]. All the information advised that the small-molecule TTQF-SO3 has particularly bone-binding affinity to efficiently visualize the entire skeletal system in vivo, which has the potential for real-time intraoperative bone imaging.
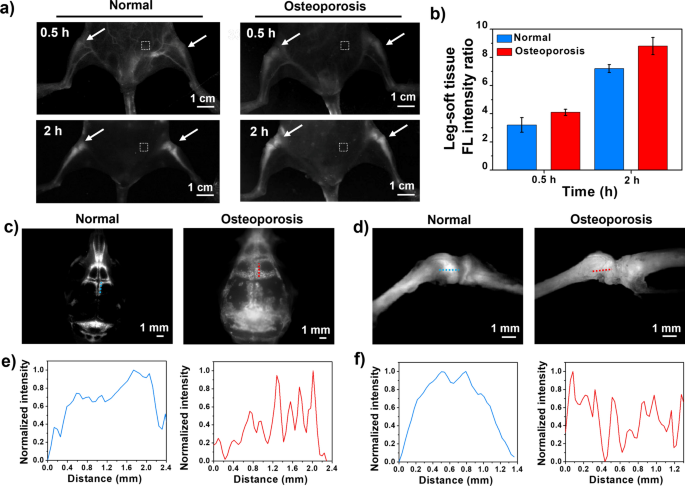
In view of the excellent bone imaging high quality of TTQF-SO3 in regular mice, osteoporosis mannequin mice have been chosen for additional evaluated its NIR-II bone-imaging. TTQF-SO3 (100 μL, 1.0 mg mL−1) was injected into regular mice (n = 3) and osteoporosis mice (n = 3) by the tail vein, after which NIR-II FI have been taken at totally different time factors (Further file 1: Fig. S14). As exhibited in Fig. 4a, after 30 min injection, TTQF-SO3-based NIR-II FI exhibited vital variations between the legs of osteoporosis mice and regular mice. Inside 2 h after injection, the SBR worth within the legs of osteoporosis mice was increased than that of regular mice from starting to finish, and the standard of NIR-II FI was considerably improved (Fig. 4b). The upper accumulation of TTQF-SO3 in osteoporosis mice could also be attributed to osteoclast exercise. These osteoclast precursors belong to the monocyte/macrophage class and have comparable phagocytosis capabilities, due to this fact, TTQF-SO3 might be simply collected into bone, which is in keeping with mobile uptake information.
a NIR-II FI of regular mice and osteoporosis mice in supine place have been obtained at 0.5 and a pair of h after injection, respectively. Imaging parameters: 1300 filter, publicity 3000 ms. Dotted field in white: smooth tissue. b Corresponding leg-tissue fluorescence sign ratio of the 2 teams. c NIR-II FI of skulls in regular (left) and osteoporotic mice (proper). Imaging parameters: 1300 filter, publicity 500 ms. d NIR-II FI of shanks in regular (left) and osteoporotic mice (proper). e, f Corresponding normalized FL depth distribution alongside yellow/crimson strains in corresponding figures c and d. Error bars, imply ± SD (n = 3)
After eradicating the pores and skin and viscera from osteoporotic mice, we additional carried out in vitro NIR-II FI evaluation utilizing an NIR-II microscope (Further file 1: Fig. S15). In contrast with regular mice, the osteoporotic mice had defects on the floor of their bones, notably the cranium and kneecaps (Fig. 4c, d). Additional quantitative bone evaluation in vitro confirmed that the fluorescence depth curves of the cranium and kneecap of regular mice confirmed a big broad peak, whereas these of osteoporosis mice exhibited many distinct sharp peaks (Fig. 4e, f). These outcomes advised that the bone floor of the osteoporosis mice was uneven. As well as, quantitative fluorescence alerts of skulls and shanks ex vivo have been additional evaluated (Further file 1: Fig. S16). The fluorescence depth of TTQF-SO3 in cranium and shank of osteoporotic mice elevated by roughly 3.0- and 1.3-fold, respectively, increased than that of regular mice, indicating the superior accumulation potential for osteoporotic mice.
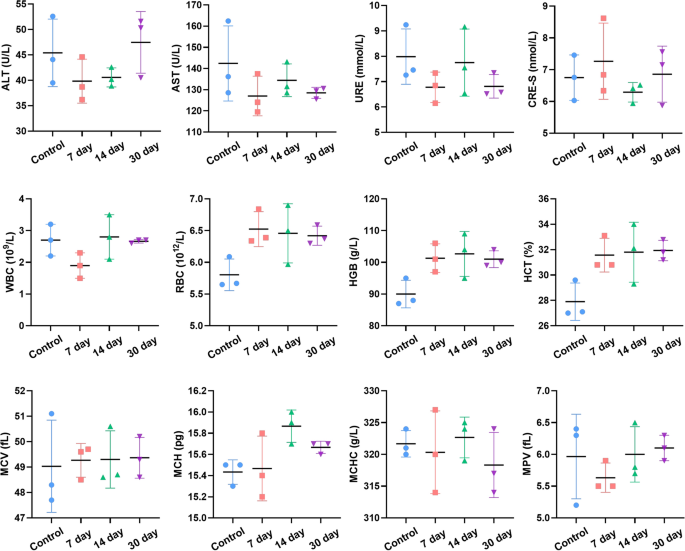
The in vivo biosafety of TTQF-SO3 was additional evaluated. As proven in Determine S17 (Supporting Info), the principle organs in vitro confirmed that TTQF-SO3 was primarily collected in liver and spleen. Furthermore, no apparent irritation or abnormalities have been noticed in main organs of mice after injection of TTQF-SO3, demonstrating that the fluorescent probe has good biocompatibility (Fig. 5; Further file 1: Fig. S18). Moreover, the conventional mouse injected with TTQF-SO3 can nonetheless observe the fluorescence alerts of the liver and bone after 21 days, indicating that the fluorophore can stay in vivo for a very long time (Further file 1: Fig. S19). Predictably, long-term retention characteristic of TTQF-SO3 in bone tissue might function concentrating on group to ship medication to main bone tumors, bone metastases, osteoporosis, osteomyelitis and so forth.