Synthesis of plasmonic hybrid nanogels (PHNs) through photo-initiated one-pot polymerization
To acquire PHNs composed of light-responsive gold nanoparticles (GNPs) and thermo-responsive polymeric nanogels, we employed a photo-initiated one-pot synthesis technique. The response combination was ready by mixing a thermo-responsive monomer (i.e., NIPAM), a number of linker molecules (Further file 1: Desk S1), a gold ion precursor (i.e., HAuCl4), and a photoinitiator (i.e., Darocur® 1173). Beneath publicity to 365 nm gentle (1.2 W/cm2), the response elements rapidly fashioned a globular construction by means of radical polymerization of the monomers and simultaneous self-integration of decreased gold ions into the polymeric community (Fig. 2a). The optimum situation for GNP synthesis was decided by altering the focus and mixing ratio of the gold precursor and photoinitiator (Further file 1: Fig. S1). After 10 min of illumination, small-sized ligand-free GNPs lower than 10 nm in measurement have been efficiently fashioned by means of ion discount by the free radicals generated from the photoinitiator [46, 47]. In the course of the polymerization of NIPAM and linkers, the GNPs that have been readily synthesized and built-in with the polymeric community through weak interactive forces between the GNP and hydrophilic moieties of the polymeric networks (i.e., –OH, –NH, and so on.) [48, 49]. Consequently, simultaneous self-integration was achieved to type hybrid nanogels composed of a polymeric community (i.e., PNIPAM) and GNPs.
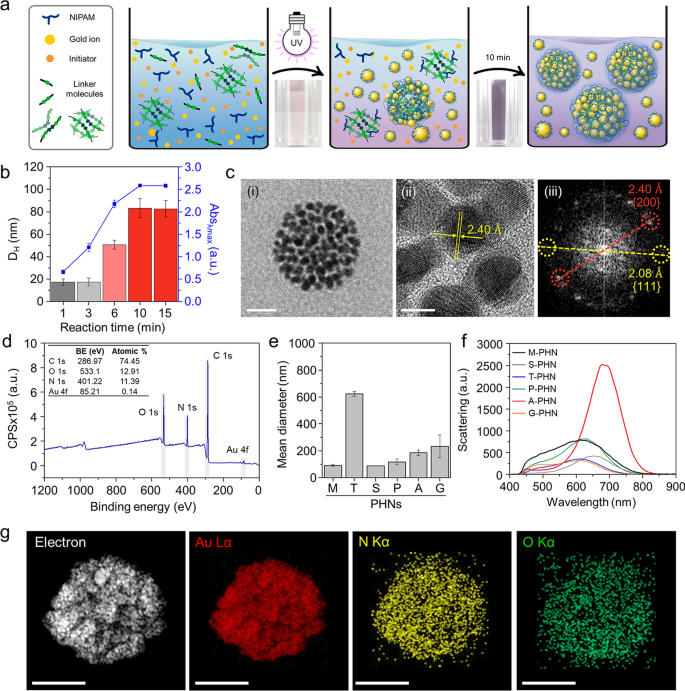
One-pot synthesis of PHNs and their physicochemical properties. a Schematic illustrations for easy one-pot fabrication process of PHNs. b Optimization of the response time by monitoring the hydrodynamic diameter and the height absorbance (λmax = 530 nm) of PHNs. c Electron microscopy (EM) evaluation of MBA-linked PHN (M-PHN). (i) Subject emission-transmission EM (TEM) picture of a single M-PHM (Scale bar: 50 nm), (ii) Excessive decision (HR)-TEM picture of the lattice construction of GNPs (Scale bar: 5 nm), and (iii) the diffraction patterns of GNPs in an M-PHN. d Extensive scan X-ray photoelectron spectra of alginate linked-PHN (A-PHN). e The hydrodynamic diameters of PHNs synthesized with numerous linker molecules. f Averaged scattering spectra of assorted species of PHNs (n = 40). g Vitality dispersive spectroscopy mapping outcomes of A-PHN utilizing HR-TEM. Elemental mapping photographs for Au, N, and O atoms (Scale bar: 100 nm)
The response time for the synthesis of PHNs was optimized by monitoring the hydrodynamic diameter and absorbance on the peak at every time level (Fig. 2b). After sampling small aliquots, an equal quantity of deionized (DI) water was added to the merchandise to terminate the free radical-mediated response. When N,N′-methylene bisacrylamide (MBA) was used as a linker molecule, the scale of the ensuing MBA-linked PHN (M-PHN) quickly elevated and reached a plateau inside 10 min. In comparison with the early-stage aliquots, the matured M-PHNs exhibited a monodisperse peak (Further file 1: Fig. S2). The diameters of the PNIPAM nanogels with out GNPs elevated to 400 nm because the response time elevated (Further file 1: Fig. S3); nonetheless, the M-PHNs reached 80 nm inside the similar response time. A distinction was noticed within the development sample between that of PNIPAM nanogel and PHNs; this might be attributed to the impact of the consumption of radicals throughout GNP formation on the expansion of the PHNs. Furthermore, the absorbance spectra of the PHNs broadened and shifted to an extended wavelength in contrast with that noticed within the response with gold ions solely (Further file 1: Fig. S4). Thus, GNPs within the M-PHNs have been well-incorporated into the polymeric networks through the response. The ensuing M-PHNs exhibited comparatively good stability beneath repetitive cycles of thermal stress than that of the PHNs with out linker molecules (Further file 1: Fig. S5). This means that GNPs bolstered the structural stability of the PHNs by being anchored to the polymeric networks and regulating the expansion kinetics of the nanogels.
The TEM photographs in Fig. 2c(i) present the consultant morphology of the M-PHNs exhibiting GNPs embedded into the globular hybrid nanogel construction. As proven within the high-resolution picture (Fig. 2c(ii)), sub-10 nm GNPs have been assembled with a small hole. The standard lattice size and diffraction patterns for the assembled GNPs revealed a powerful correspondence with the metallic GNPs, which have a face-centered-cubic side (Fig. 2c(iii)) [50]. Not like the GNPs, the polymeric community of the PHNs was not seen within the TEM photographs, which could be attributed to the thinness of the layer and low electron density. X-ray photoelectron spectroscopy (XPS) measurements have been carried out on the outermost layer of the PHNs to characterize the polymeric layer of the PHNs with precision. The wide-scan survey spectrum of the PHNs confirmed peaks equivalent to C1s, N1s, O1s, and Au4f on the attribute binding energies (Fig. 2d). The relative atomic share of Au was nearly indiscernible as a result of the floor of the GNPs was largely coated with skinny polymeric layers. Nonetheless, the metallic Au0 peaks at 88.28 eV and 84.58 eV have been measured from the slender scan (Further file 1: Fig. S6). This means that the Au ions have been efficiently decreased to metallic Au throughout polymerization. Furthermore, the height shifted barely to increased binding vitality, indicating that the GNPs have been embedded within the polymeric community by means of weak interactive forces (e.g., Van der Waals, dipole–dipole, induced dipole, and so on.) [46, 47, 49, 51, 52]. Within the C1s area, the spectrum was deconvoluted into three peaks (i.e., 284.78 eV for the C–C peak, 286.2 eV for the C–N peak, and 287.48 eV for the C=O), which point out the presence of PNIPAM constructions on the Au floor. As well as, the existence of PNIPAM was double-checked by observing at 532 eV for the C=O peak within the O1s spectrum. These XPS outcomes point out that the GNPs have been efficiently entrapped inside the skinny PNIPAM-based nanogels.
For the reason that light-responsive property is carefully associated to the absorption and scattering cross sections [53], the mixing density of GNPs within the PHN construction or the diameters of PHN must be additional optimized. For this function, 5 sorts of different linker molecules, tryptophan (T), sucrose (S), polyethylene glycol diacrylate (P), alginate (A), and gelatin(G) have been examined to manage the density of the GNPs and the scale of the PHNs. Determine 2e exhibits the distinction within the diameter of the synthesized PHNs, which could be attributed to the completely different physicochemical properties of the linker molecules comparable to hydrodynamic diameters, purposeful teams, and the presence of hydrophilic pockets. The sugar-ring construction is understood to stabilize the GNPs owing to their excessive hydrophilicity; therefore, the structural stability of PHNs may be improved by embedding GNPs into extra polysaccharides [47, 51, 52]. As anticipated, each the density of the GNPs and the diameter of the PHNs relied on the linker molecules (Further file 1: Fig. S7). Within the case of M-PHN, P-PHN, and A-PHN, smaller GNPs (i.e., lower than 10 nm) tended to be extra carefully packed within the constructions. Specifically, the PHN fabricated with alginate because the linker (i.e., A-PHN) exhibited the best density of the small GNPs. Consequently, the absorbance peaks shifted to longer wavelengths upon altering the linker molecules (Further file 1: Fig. S8a). Though the PHNs with large-sized GNPs (e.g., T-PHN and S-PHN) had red-shifted absorption bands, they confirmed poor colloidal stability (Further file 1: Fig. S8b), whereas the A-PHN, P-PHN, and G-PHN have been steady after in a single day storage at room temperature (i.e., 25 °C). Furthermore, A-PHN exhibited the best scattering peak at roughly 700 nm (Fig. 2f), which might be attributed to the big optical cross-sectional space induced by the excessive density of GNPs. The molecular weight (MW) of A-PHN was estimated by measuring static gentle scattering. As proven in Further file 1: Fig. S9, the measured MW of A-PHN was discovered to be 1.04 × 107 Da, whereas these of PNIPAM and PNIPAM-alginate exhibited 2.53 × 105 and 4.24 × 106 Da, respectively. The comparatively excessive MW of the A-PHN is attributable to the GNP integration into the polymeric nanogels. Furthermore, lyophilized A-PHN exhibited superior water solubility of as much as 30 mg/mL (Further file 1: Fig. S10). Based mostly on these outcomes, A-PHN was chosen for additional characterization and drug supply functions.
To confirm the existence of the alginate, we used calcium ions, that are well-known gelation linkers of alginate-based hydrogels and type a calcium-alginate egg-box construction [54]. As proven in Further file 1: Fig. S11, alginate-containing dispersions (i.e., A-PHN and alginate solely) fashioned hydrogels within the presence of Ca2+, whereas gel formation was not noticed for M-PHN. The Ca2+-induced formation of the purple-colored gel noticed within the case of the A-PHN indicated that alginate molecules have been efficiently integrated into the GNPs and PNIPAM community as a result of the alginate-only hydrogel exhibited a white coloration. Elemental mapping (Fig. 2g) and energy-dispersive X-ray spectroscopy outcomes (Further file 1: Fig. S12) for A-PHN additionally confirmed the profitable integration of every element. These outcomes confirmed that high-density GNPs are embedded into alginate-linked PNIPAM nanogels, and the ensuing A-PHN possesses a big optical cross-sectional space to the incident gentle.
Native warmth era by photothermal conversion of GNPs within the PHNs
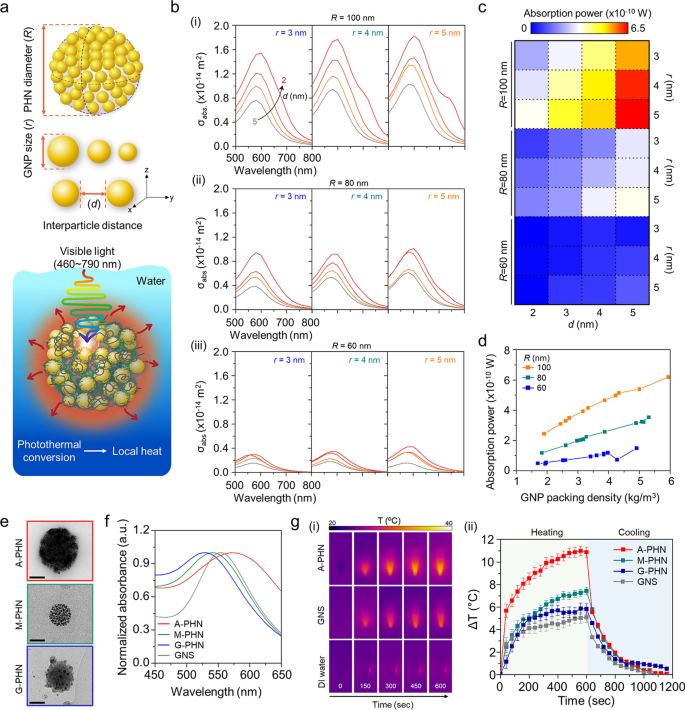
A wave optics simulation was carried out to foretell the native warmth era from the PHNs beneath gentle illumination by calculating the absorption energy of GNPs within the PHNs. First, it was thought of {that a} single PHN construction was immersed in water, and the sunshine at an depth of three.5 W/cm2 was repeatedly irradiated at wavelengths from 460 to 790 nm. For this modeling, the GNPs have been organized at common intervals primarily based on the GNP measurement (r) and interparticle distance between the GNPs (d) on the differential PHN diameter (R) (Fig. 3a and Further file 1: Fig. S13). The optical properties of the PHN construction have been evaluated at completely different values of r (3, 4, and 5 nm), d (2, 3, 4, and 5 nm), and R (60, 80, and 100 nm). The variety of GNPs was set to the utmost quantity that might be enclosed within the PHN. Determine 3b exhibits the absorption cross-section (σabs) of the PHN based on the wavelength of the incident gentle. The spectrum of σabs varies based on r and d. When the radius of the PHN was 100 nm, σabs elevated as r elevated, and d decreased. These outcomes present an identical tendency as R modified from 80 to 60 nm. The absorption energy was calculated at a wavelength of 550 nm, the place PHN confirmed the utmost σabs. As proven in Fig. 3c, the absorption energy additionally elevated as r elevated, and d decreased when R = 100 nm. Furthermore, the absorption energy at 550 nm elevated with the GNP packing density within the PHNs (Fig. 3d). Subsequently, a small variety of GNPs with bigger r values present increased absorption energy than numerous GNPs with smaller r when the interparticle distance was the identical. Because of this, the elevated density of the proximate GNPs within the massive PHNs induced extra intensive warmth era.
Analysis of native warmth era from the photothermal conversion of GNPs in PHNs beneath gentle illumination. a Schematics of computational simulation parameters (i.e., R, r, and d) and modeling surroundings. b Computational simulation for the absorbed cross-section of PHNs based on the diameters of single GNP (r), from left (r = 3) to the fitting (r = 5) for (i) R = 100, (ii) R = 80, and (iii) R = 60. c Absorption energy map profile for the diploma of native warmth era for numerous values of R, r, and d. d GNP packing density-dependent absorption energy plots at 550 nm by altering the R. e Consultant TEM photographs of the PHNs with numerous integration densities of GNPs (Scale bar: 50 nm). f Normalized absorbance spectra of the PHNs and GNS. g Monitoring of warmth era by 100 µL PHNs and 80 nm GNS. (i) Infrared thermal photographs. (ii) Thermal elevation curves with heating and cooling interval (n = 3)
To experimentally validate this prediction, three sorts of PHNs (i.e., M-PHN, A-PHN, and G-PHN), which have completely different GNP densities, have been ready with the identical focus (i.e., 1 mg/mL, optical density = 1OD at peak) as proven in Fig. 3e. Contemplating the same measurement and absorption peak wavelength of the PHNs, an 80-nm gold nanosphere (GNS) was used as a management to judge the photothermal conversion effectivity of the PHNs (Fig. 3f). As proven in Fig. 3g, the answer temperature of the colloidal A-PHN drastically elevated by 11 °C on publicity to a 532 nm laser (3.5 W/cm2). Notably, the temperature increment relied on the density of the GNPs (i.e., A-PHN > M-PHN > G-PHN). Specifically, the warmth increment degree was twofold increased for the A-PHN, which had high-density GNPs, in contrast with that of the GNS. Moreover, the photothermal conversion effectivity (η) of the GNS and PHNs was calculated to quantitatively examine the warmth generated by the colloids [30, 35]. The speed of warmth switch was measured by eradicating the sunshine supply and monitoring the lower in temperature (see Further file 1 for detailed calculations). From the calculations, η was discovered to be 15.69, 8.54, and three.99% for A-PHN, M-PHN, and GNS, respectively (Further file 1: Fig. S14). The next η for A-PHN than for M-PHN signifies that the packing density of GNPs within the PHN is essential for successfully producing warmth in response to gentle. For lyophilized PHN powder, temperature increments prominently occurred as much as 80 °C, even beneath publicity to laser at a comparatively low energy density (532 nm, 0.8 W/cm2). This may be attributed to a high-density state within the powder than within the colloidal state of the PHNs (Further file 1: Fig. S15). Temperature elevation increased than 20 °C was achieved within the A-PHN colloids by growing the illumination time to 1800 s (Further file 1: Fig. S16) utilizing a industrial LED (0.8 W/cm2), which emitted gentle with a broad wavelength (480–700 nm).
Photothermally-driven conformational modifications of PHNs
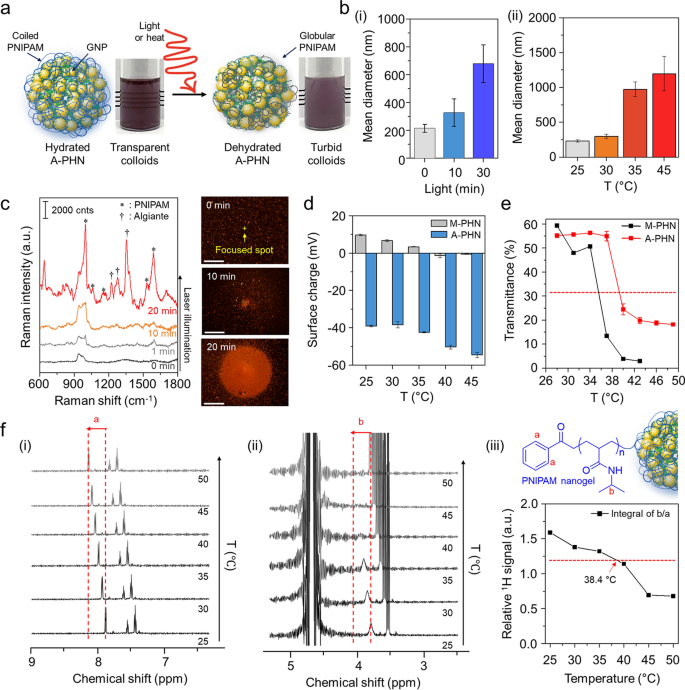
Because the resonant laser was able to growing the answer temperature by greater than 10 °C inside 10 min, we supposed that it was adequate to induce the conformational modifications within the PNIPAM community at A-PHN. The warmth- or light-responsive properties of hydrated A-PHNs in an aqueous resolution have been evaluated by growing the answer temperature and light-weight publicity time. Beneath appropriate stimulation, the A-PHN construction grew to become unstable owing to the elevated hydrophobicity of the PNIPAM aspect chains, which led to interparticle aggregation (Fig. 4a). The hydrodynamic diameters of A-PHNs steadily elevated upon publicity to each gentle (Fig. 4b(i)) and warmth (Fig. 4b(ii)). This means that both native or international warmth can induce conformational modifications within the A-PHNs. For the reason that hydrophilic PNIPAM chains dehydrate as the answer temperature exceeds the LCST [37, 38], their hydrophobic isopropyl department and spine type a globular construction owing to the hydrophobic interplay, which leads to the agglomeration of A-PHNs in colloids.
Investigation of photothermally-driven conformational modifications in PHNs. a Schematics for dehydration process of A-PHN beneath the stimuli (i.e., gentle or warmth). b Hydrodynamic diameters of A-PHN dispersion based on altering (i) gentle publicity time and (ii) resolution temperature. c In-situ Raman measurements of A-PHN through the steady laser illumination, and dark-field photographs of assembled A-PHN at one spot (Scale bar: 20 µm). d Floor prices of A-PHN and M-PHN by growing the answer temperature. e Monitoring of transmittance utilizing A-PHN and M-PHN by growing the answer temperature. f Temperature-dependent 1H-NMR of A-PHN dispersed in D2O. (i, ii) Cropped spectra equivalent to PNIPAM nanogel-specific bonds denoted as a and b. (iii) The temperature-dependent relative integrals of the everyday proton alerts from spectra
To verify the conformational modifications of the PNIPAM items in A-PHN, Raman measurements have been carried out to differentiate between the hydrated and dehydrated standing. Because the alginates have been linked with PNIPAM within the A-PHN, the shrinkage of the PNIPAM chain might be preserved with the introduction of Ca2+ within the turbid state (i.e., dehydrated A-PHN gel). As proven in Further file 1: Fig. S17, the dehydrated A-PHN gel offered fingerprint peaks for alginate (ν = 1240, 1350, and 1440 cm−1) and hydrophobic moieties of PNIPAM (i.e., C–C ring stretching and respiratory, ν = 980, 1050, and 1580 cm−1), in contrast to the hydrated gel. This may be interpreted to point that the alginate-linked PNIPAM community within the PHN was proximate to the GNPs by conformational modifications induced because of dehydration. In Fig. 4c, the dark-field scattering photographs present the spatially-controlled formation of agglomerates beneath gentle illumination. With growing gentle publicity time, the scale of the agglomerate elevated, and the ensuing Raman sign for the A-PHN dynamically modified to a spectral sample much like that of the dehydrated gel. Because of this the photothermal conversion of GNPs can induce conformational modifications within the thermo-responsive PNIPAM at A-PHN. Moreover, the change within the floor cost with growing temperature clearly exhibits proof of such modifications within the PNIPAM construction on the floor of A-PHN (Fig. 4d). The floor cost of A-PHN was initially unfavourable (c.a. − 39 mV) owing to the negatively charged alginate, and it modified to a extra unfavourable worth of − 54 mV as the answer temperature elevated to 45 °C. Within the case of M-PHN, the identical tendency towards unfavourable prices was noticed. Subsequent investigations centered on discovering an optimum temperature vary for the conformational modifications in PHNs. Based mostly on the change in transmittance and turbidity of the colloidal PHNs with growing temperature, LCST values of roughly 38.4 °C and 35.6 °C for the A-PHN and M-PHN, respectively, have been obtained (Fig. 4e). These temperatures have been increased than that obtained for PNIPAM-based polymers on the whole (i.e., 32–33 °C). That is most likely because of extra intermolecular interactions (i.e., hydrogen bonds) between PNIPAM and alginate or GNPs in A-PHN. Furthermore, the 1H-NMR research utilizing A-PHN, which is dissolved to 10 mg/mL in D2O, confirmed a temperature-dependent peak shift to downfield, which is robust proof of the conformational modifications within the PNIPAM nanogel (Fig. 4f and Further file 1: Fig. S18) [55]. Since these modifications of A-PHN happen near 38.4 °C, which is an simply achievable vary by means of the photothermal conversion of GNPs, photothermally triggered spatiotemporal controllability of A-PHN within the mobile supply may be simply completed.
Photothermally-driven spatiotemporally managed drug supply utilizing A-PHN
To confirm the managed drug launch by photothermally pushed conformational modifications of the A-PHN (Fig. 5a), two mannequin medication, doxorubicin (dox) and paclitaxel (ptx), have been loaded into the A-PHN through the calcium-induced gelation technique. Briefly, 1 mg/mL A-PHN was combined with every drug resolution (i.e., 200 µM dox and 1 µM ptx) and subsequently added 10 mM CaCl2. Then, they incubated for two h to test the drug-loaded gel formation. After being remoted from free medication, the precipitates have been re-suspended to water. The loading effectivity of dox was calculated to be 60% (v/v) based on the usual curve for dox absorbance (Further file 1: Fig. S19). As proven in Fig. 5b, the loaded dox was launched sooner from the A-PHN within the presence of sunshine (LED, 0.8 W/cm2). And, the discharge kinetics have been dependent upon the sunshine energy density (Further file 1: Fig. S20). Furthermore, the discharge profile drastically elevated beneath acidic circumstances, which resulted from the loosened polymeric community at low pH (Further file 1: Fig. S21) and the synergistic impact of protonated dox to spice up the discharge [56]. Moreover, photothermally induced drug launch was monitored utilizing the intrinsic Raman alerts of dox (Fig. 5c). In line with the illumination time, the Raman alerts of dox emerged steadily, whereas the identical peaks have been additionally noticed within the drug-loaded A-PHN itself (see Fig. 4c). This attribute of the structural modifications in A-PHN additionally occurred following the loading of dox with calcium ions. Furthermore, a sequence of Raman spectra have been obtained to test the temporal controllability of drug launch from A-PHNs beneath discrete gentle illumination (532 nm laser, 3.5 W/cm2). Determine 5d(i) exhibits the Raman alerts obtained alternatively within the presence and absence of laser illumination. The launched dox alerts drastically elevated initially through the “laser on” situation (Fig. 5c and Further file 1: Fig. S22), whereas solely minimal alerts have been noticed through the “laser off” situation. The rise within the Raman sign slowed as the quantity of remaining dox contained in the A-PHN decreased after the primary sequence (Fig. 5d(ii) and (iii)). This clearly demonstrates the temporal controllability of drug launch in response to gentle. The colloidal stability of the A-PHNs was evaluated by measuring imply diameters in numerous organic media together with PBS, DMEM, and RPMI. As proven in Further file 1: Fig. S23, the change in diameters was negligible for 7 days, indicating the excessive stability of the A-PHN in organic media. Furthermore, the MTT assay was performed to verify the cytotoxicity of A-PHN itself and the mobile impact from photothermal conversion. As proven in Further file 1: Fig. S24, no vital cytotoxicity of nanogels and A-PHNs even at excessive concentrations was noticed earlier than gentle publicity.
Photothermally-driven spatiotemporally managed drug supply utilizing A-PHN. a Schematic mechanisms of drug launch from a dehydrated A-PHN by gentle illumination. b Cumulative dox launch beneath gentle publicity by measuring the absorbance of free dox utilizing completely different pH circumstances. c Time-coursed Raman spectra with steady gentle illumination. d Monitoring the Raman spectra of launched dox beneath temporally managed gentle modulation. (i) Schematics for the discrete gentle illuminating experiments. (ii) Switchable launch profile of dox@A-PHN beneath laser on/off circumstances. (iii) Cumulative dox sign kinetics beneath discrete gentle illumination (n = 6). e Remark of light-triggered dox supply utilizing A-PHN. (i) Fluorescent colocalization photographs of launched dox inside the nucleus. The pink coloration signifies the dox, and the blue signifies the nucleus. The dimensions bar is 50 µm. (ii) Colocalization diploma monitoring by Pearson’s coefficient with gentle publicity time (n = 3). f Cell viability take a look at utilizing MTT assay of A-PHN and dox@A-PHN with/with out gentle publicity (n = 5)
Dox is well-known for particularly concentrating on the cell nucleus; due to this fact, the launched medication from the A-PHN would journey quickly into the nuclei. To visualise this, dox@A-PHN (i.e., 80 µM dox) was used to deal with A375P melanoma cells. The cells have been positioned beneath an LED (0.8 W/cm2) for predetermined intervals (i.e., 0, 1, 10, and 30 min). Non-light illuminating teams have been positioned at midnight to keep away from undesired leakage of dox from the A-PHN. Because of this, colocalization of dox inside the nuclei was extra noticeable within the cells within the 30-min gentle publicity group than within the cells of the management group that have been incubated at midnight (Fig. 5e(i)). To quantify the supply effectivity, Pearson’s correlation coefficient (okay) was used to look at the diploma of colocalization between dox and Hoechst. As anticipated, this worth tended to extend in a time-dependent method from 0.07 (at 0 min) to 0.645 (30 min). The worth of the management group was considerably decrease than that of the light-exposure teams (Fig. 5e(ii) and Further file 1: Fig. S25). A cytotoxicity take a look at was carried out beneath the identical circumstances to additional verify drug supply efficacy. To keep away from unwanted side effects on the cells from the remaining dox@A-PHN, the incubated cells have been fastidiously washed and changed with contemporary media. Since dox doesn’t kill the cells instantly, extra incubation was offered in a single day earlier than assaying. For the cells handled with dox@A-PHN beneath 30 min of sunshine illumination, a big lower (48.9 ± 4.6%) in cell viability was noticed (Fig. 5f). Notably, no vital cell dying was noticed within the management teams, together with gentle publicity solely with out dox@A-PHN (101.3 ± 5.9%), dox@A-PHN at midnight (97.0 ± 1.8%), and A-PHN solely (95.7 ± 4.8%) teams with and with out gentle publicity.
Photothermally facilitated the endosomal escape of mobile internalized A-PHN
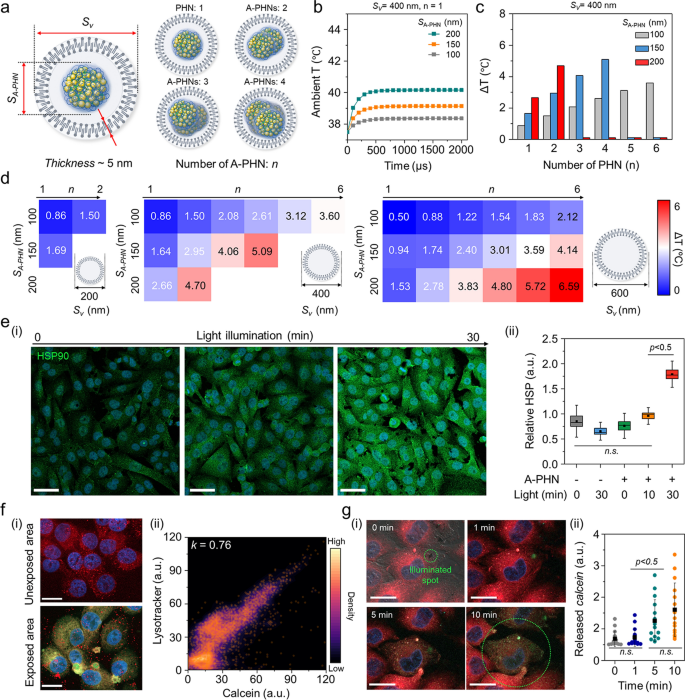
For a extra nuanced understanding of the subtle drug supply mechanism, we investigated the temperature distribution inside vesicles containing A-PHNs. By observing the fluorescent and dark-field scattering photographs, we confirmed that A-PHN with 150–200 nm in diameter might be internalized into cells through the endocytosis pathway [15, 16]. As proven in Further file 1: Fig. S26, the diameters of vesicles together with A-PHNs ranged between 200 and 800 nm and are visualized as pink fluorescent dots (i.e., endo-lysosomes) and orange scattering dots (i.e., A-PHN-containing vesicles). The broad distribution of vesicle sizes may be attributed to the extra packing course of through the proximate vesicle fusion within the cytosol after internalization. Based mostly on the experimental statement of endocytosis, a simulation was carried out to foretell the temperature profiles by altering the variety of A-PHNs within the endocytic vesicle beneath the sunshine. As proven in Fig. 6a, the simulation area consisted of a vesicle that enclosed the A-PHNs. The outer layer of the vesicle was a lipid bilayer membrane with a thickness of 5 nm, and the within of the vesicle was assumed to be stuffed with water. We performed a simulation by various the scale of the vesicles (Sv) and A-PHNs (SA-PHN) and the variety of encapsulated A-PHNs (n). Within the simulation, numerous Sv (200 nm, 400 nm, and 600 nm), SA-PHN (100 nm, 150 nm, and 200 nm), and n (1 to six) have been utilized. The temperature modifications contained in the vesicles have been calculated utilizing the utmost absorption energy obtained from the wave optics simulation as the warmth supply for the heat-transfer simulation. The preliminary ambient temperature was set to 37.5 °C, which is the widespread incubation temperature for mobile experiments. The common temperature contained in the vesicles quickly elevated within the presence of A-PHN (< 500 µs) and might be modulated based on the modifications in parameters (Fig. 6b and Further file 1: Fig. S27). For instance, the imply temperature elevated from the preliminary temperature of 37.5 °C to 42.6 °C when n = 4, Sv = 400 nm, and SA-PHN = 150 nm (Fig. 6c). The results of the warmth switch simulation (Fig. 6d) confirmed that A-PHNs within the endocytic vesicle may generate adequate temperature for vesicle rupture (i.e., ΔT > 4 °C) [57,58,59]. These outcomes point out that spatially managed drug supply is feasible after the development of endocytic vesicle development.
Pinpoint cytosolic manipulation and spatially managed drug launch because of light-triggered vesicle rupture in a single cell. a Schematics of the simulation area encompass the endocytic vesicle that encloses the A-PHNs. b Computational simulation for warmth elevation profile utilizing Sv = 400 nm and n = 4 by altering the scale of single SA-PHN within the vesicle. c Imply temperature modifications of the vesicle with Sv = 400 nm. d Temperature shift expectation plot by altering the quantity (n), and measurement (SA-PHN) of PHNs within the various-sized vesicle (Sv). e Monitoring the mobile response (i.e., HSP expression) based on gentle illumination. (i) Fluorescence photographs for the elevated HSP beneath the delicate LED (0.8 W/cm2). The dimensions bar is 50 µm. (ii) Comparability of relative HSP depth (n = 5) primarily based on illuminating time. f Spatially managed supply utilizing calcein-encapsulated A-PHN by photothermally-driven vesicular rupture. (i) Fluorescence photographs of endo-lysosome and launched calcein from the A-PHN on the unexposed and uncovered areas (Scale bar: 20 µm). (ii) Colocalization density plot between lysotracker and calcein (orange dots point out the colocalization spots). g Actual-time monitoring of calcein leakage on the single cell. (i) Time-coursed confocal photographs by illuminating the laser (550 nm with 3.5 W/cm2). Purple coloration signifies the endo-lysosomes, and inexperienced signifies the launched calcein. The dimensions bar is 10 µm. (ii) Launched calcein depth profiles within the single cell based on the sunshine publicity time (n.s. point out the non-significance)
To verify the simulation outcomes experimentally, the cytosolic response was first monitored by making use of laser illumination (532 nm, 3.5 W/cm2) to a vesicular A-PHN (Further file 1: Fig. S28). After the laser remedy, bubbles have been generated instantly (< 1 s) across the A-PHN, which irreversibly destroyed the cytoskeleton. This additionally signifies {that a} excessive degree of warmth was generated across the vesicles [60, 61]. For the reason that cells keep homeostasis beneath stress circumstances, the photothermal-driven cytosolic temperature modifications would additionally affect mobile elements. For instance, owing to the native warmth era in cells, warmth shock proteins (HSPs) may be upregulated by gentle publicity within the presence of A-PHN. After incubating the A-PHNs in A375P cells for 4 h, low energy LED (0.8 W/cm2) was used to light up the cell. The extent of HSPs markedly elevated within the A375P cells with internalized A-PHN based on the illumination time (Fig. 6e(i)). As proven in Fig. 6e(ii), a drastic improve within the HSP depth was noticed within the A-PHN-internalized A375P cells with statistical significance inside 30-min of sunshine publicity. That is in keeping with the statement offered in Fig. 5e, which signifies that the internalized A-PHNs may manipulate mobile responses by producing warmth from the vesicles.
To successfully ship medication to focused mobile websites, internalized drug-loaded carriers should escape from endocytic vesicles by staining the endosomes with wheat germ agglutinin, which binds to glycoproteins of the cell membrane, mobile internalization was validated for A-PHN by means of the receptor-mediated endocytic trafficking pathway [62, 63]. As proven in Further file 1: Fig. S29, a lower in inexperienced fluorescence indicating endosomes was prominently noticed within the late endosome areas. That is properly supported by the simulation consequence (see Fig. 6d) displaying that the endosomal rupture preferentially happens in vesicles containing a number of A-PHNs after the development of endocytic vesicle development. To verify the drug launch from vesicles, a membrane-impermeable fluorescent dye (i.e., calcein, 5 mM in DMSO) was encapsulated into the A-PHN and delivered to the cells [20, 21]. As proven in Fig. 6f(i), the inexperienced fluorescent alerts equivalent to the launched calcein have been unfold into the entire cytosol within the light-exposed area of the cell (550 nm laser, 3.5 W/cm2), whereas solely pink dots (i.e., lysotracker, DND-99) have been noticed within the unexposed space. The calcein alerts launched into the cytosol extremely overlapped with the lysotracker alerts after full unfold (okay = 0.76, Fig. 6f(ii)), which signifies spatially managed drug supply solely within the light-exposed area. Furthermore, we noticed widespread cytosolic distribution of calcein inside 10 min of sunshine remedy in a single cell (Fig. 6g). This reveals that pinpoint cytosolic manipulation may be achieved rapidly and A-PHN can selectively affect the vesicular membrane by native warmth era for spatiotemporally managed drug supply.
Validation of enhanced drug supply effectivity of PHNs utilizing mobile 3D spheroids
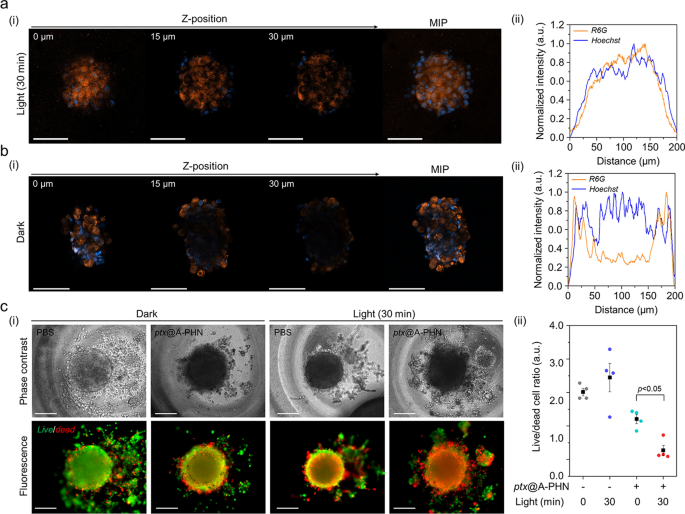
To additional study the improved drug supply effectivity utilizing A-PHNs, a three-dimensional (3D) spheroid of cells, which mimics the microenvironments of the physiological system, was utilized. The supply effectivity was evaluated primarily based on the drug penetration depth and the ratio of dwell/useless cells. The penetration depth profiles have been evaluated utilizing a fluorescent dye (i.e., rhodamine 6G, R6G) loaded into the A-PHN (i.e., R6G@A-PHN, 100 nM R6G). The outcomes confirmed that R6G was efficiently subtle into the deeper areas of the spheroid within the light-exposed group (Fig. 7a, 532 nm laser, 3.5 W/cm2), whereas the spheroid at midnight exhibited a restricted penetration depth (solely observable close to the outer floor, Fig. 7b). That is attributable to the era of a temperature gradient from the A-PHN beneath gentle illumination, which might induce the fast diffusion of drug molecules launched from the A-PHN. Furthermore, for the reason that medication launched from the A-PHN can simply contain cell–cell communications to adjoining cells within the spheroid [64, 65], deeper penetration of the medication can be achievable.
Enhanced drug supply effectivity of the PHNs, validated utilizing 3D mobile spheroids. Monitoring the drug penetration depth into the spheroids utilizing R6G@A-PHN beneath a gentle publicity and b darkish circumstances. (i) Z-stack confocal photographs and most depth projection (MIP) picture of a single spheroid. Scale bars point out 100 µm. The orange coloration signifies the R6G, and the blue coloration represents the nucleus within the spheroid. (ii) XY-plane profiles of fluorescent distribution within the spheroid. c Analysis of supply efficacy towards 3D spheroid utilizing the launched drug (i.e., ptx). (i) Dwell/useless cell photographs (Scale bar: 200 µm) and (ii) comparisons of the dwell/useless cell ratio from particular person spheroids (n = 5)
Within the dwell/useless cell assay, the non-fluorescent anticancer drug paclitaxel (i.e., ptx) was used to keep away from the overlapping of fluorescence through the assay. Previous to conducting spheroid experiments, phenotypic modifications within the two-dimensional (2D) cell tradition mannequin have been assessed by checking the ptx@A-PHN to find out whether or not the launched medication labored precisely (Further file 1: Fig. S30). Ptx-induced apoptotic nuclear fragmentation in A375P cells was noticed in each ptx-and ptx@A-PHN (with gentle publicity)-treated teams, whereas no change was noticed within the A-PHN remedy group. As ptx influences mitotic spindle meeting, chromosome segregation, and cell division by inhibiting microtubule meeting [66], nuclear fragments generally is a results of the motion of intracellular ptx launched from A-PHN. To guage drug supply efficacy, the dwell and useless cells of the spheroid have been stained after incubation of the spheroid with ptx@A-PHN beneath gentle publicity (532 nm laser, 3.5 W/cm2) or at midnight. As proven in Fig. 7c(i), the proportion of the red-colored useless cells within the spheroid was higher solely within the light-illuminated group handled with ptx@A-PHN, indicating that the supply efficacy was enhanced by the photothermally triggered launch and fast diffusion of ptx from A-PHN. To quantify this, the fluorescent alerts of each dwell and useless cells have been collected and the proportion of dwell/useless cells was calculated (Fig. 7c(ii)). Within the case of the 30-min gentle publicity group utilizing ptx@A-PHN, the ratio drastically decreased to 85.38% in contrast with that of the unexposed group. Collectively, medication launched from the light-responsive A-PHNs have been noticed to diffuse into the deeper area of the spheroid, killing extra cells.