Chinese language drug 3D printing agency Triastek has concluded its First-in-Human (FIH) research of its new 3D printing drug, dubbed T21. This drug is designed to deal with reasonable to extreme ulcerative colitis (UC).
In line with the research’s imaging findings, T21 tablets show correct supply and managed launch to the colon, the place the drug is meant to take impact. The tablets are produced utilizing the corporate’s Soften Extrusion Deposition (MED) 3D printing course of, which permits for exact regulation of the drug’s launch within the gastrointestinal tract. Consequently, this know-how ensures a extra centered and efficient drug supply mechanism.
“The primary human research information with T21 verifies the exact colon supply functionality of the MED course of, and this platform is poised to turn into the novel drug supply system of selection for brand spanking new colon-targeted merchandise with both native efficacy or systemic absorption,” stated Professor Xiaoling Li, Co-Founder and Chief Scientific Officer of Triastek. “We hope to proceed showcasing how Triastek’s 3D printing processes can convey technical options to pharmaceutical firms for environment friendly product growth of optimized drug supply, in the end resulting in the flexibility to offer sufferers with extra clinically invaluable medicines.”
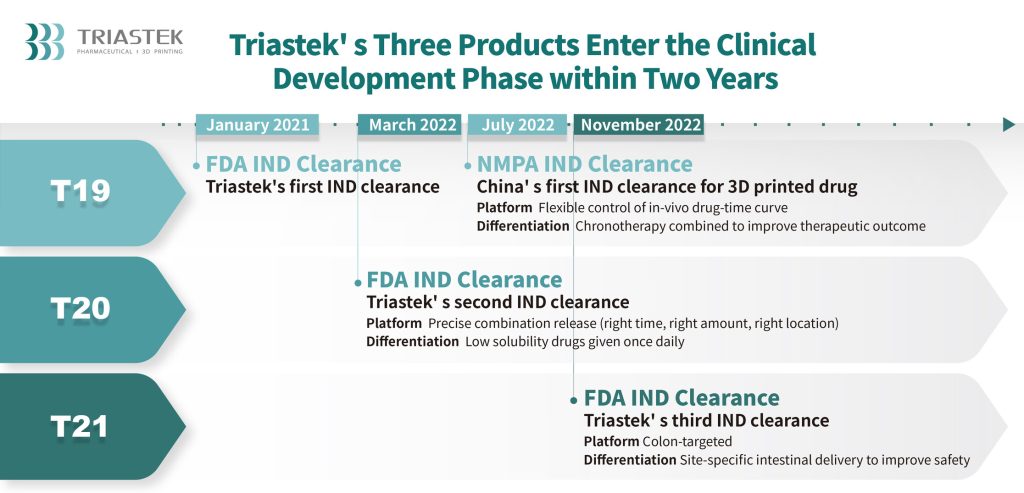
Focused and efficient drug supply
In line with the corporate, oral remedy is most popular for UC sufferers resulting from security, ache avoidance, and affected person compliance. Triastek’s T21 permits exact and focused drug supply to the gastrointestinal tract, bettering effectiveness.
T21 is the corporate’s third 3D printed drug product and serves as an oral drug comprising a complete of three layers specifically, the enteric layer, delay layer, and drug core. The enteric layer maintains the pill’s construction within the gastric acidic surroundings, making certain energetic components will not be launched prematurely. The delay layer erodes at a continuing price within the gut, attaining time-delayed drug launch. Upon reaching the colon, the delay layer totally erodes, releasing energetic components in a focused method.
T21 obtained clearance for its Investigational New Drug (IND) software from the U.S. Meals and Drug Administration (FDA) underneath the 505(B)(2) pathway, on November 18, 2022. Derived from the oral Janus kinase (JAK) inhibitor referred to as tofacitinib, T21 was authorised, main Triastek to provoke FIH analysis in Q1 of 2023. The FIH research additionally aimed to look at the transport, erosion course of, and launch website of T21 within the human physique following oral administration.
Triastek’s goal is to ascertain collaborations with pharmaceutical firms to leverage the benefits of 3D printing in remedy manufacturing. Considered one of its latest partnerships includes Boehringer Ingelheim, Eli Lilly, and Merck KGaA.
The entire addressable marketplace for 3D printed medicine
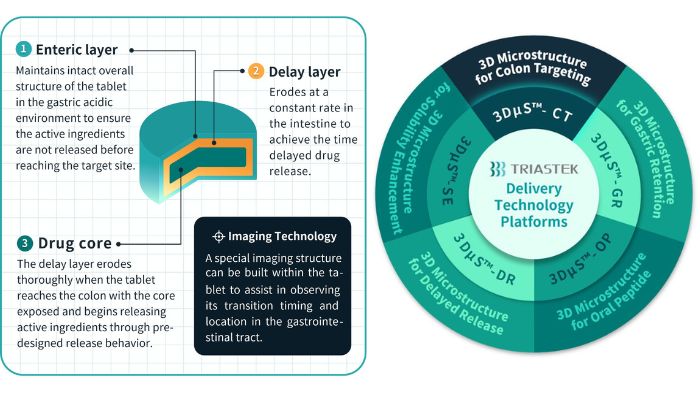
In line with Triastek, drug 3D printing know-how might be broadly used for chemical medicine, organic medicine and peptides. Triastek’s MED know-how gives end-to-end options, together with novel dosage kind design (multi-layered, honeycomb/weave and multi-compartments, and many others.), FbD product growth, and steady manufacturing at industrial scale. The corporate’s 3D printing formulation platform, 3DFbD, interprets goal Pharmacokinetic profile (PK) profiles into corresponding launch profiles. It permits exact pill design for particular, hard-to-reach GI areas just like the colon, making certain managed API launch on the desired onset time, price, and quantity.
MED know-how additionally aids in formulating problem compounds to beat biopharmaceutical deficiencies through gastro-retention and enhancing oral absorption of biologics. Triastek gives a commercial-scale MED 3D printing system able to producing as much as 50M tablets yearly. It accommodates each “blockbuster and uncommon illness merchandise,” enabling versatile manufacturing.
Moreover, Triastek notes that the pharmaceutical preparation market is split into stable and non-solid areas. 3D printing might be utilized to provide stable preparations, that are dominated by small-molecule medicine. As per a report from Consider Pharma referenced by Triastek, the worldwide drug market was $894 Billion in 2022 and is predicted to achieve $1,236 Billion in 2028. Moreover, The worldwide small molecule drug market was $433 Billion in 2022 and is predicted to achieve $549 Billion in 2028.
Professor Xiaoling Li gave a sign of how a lot of the worldwide pharmaceutical market is likely to be addressable by 3D printing. “3D printing for prescription drugs is in its infancy. Present functions of 3D printing for prescription drugs have been largely for small molecule medicine, with some exploratory research for peptides and different biomolecules within the oral supply areas through personalised dosing or mass manufacturing. Different areas of functions embrace API synthesis, implants, and drug machine mixtures. It’s untimely to estimate the share of 3D printed prescription drugs within the projected gross sales. Nonetheless, the share of 3D printed prescription drugs will steadily enhance as this know-how is taking a foothold in above talked about pharmaceutical areas,” added the Co-Founder and CSO.
3D printing know-how revolutionizes drug manufacturing
Final yr, international prescription drugs firm Eli Lilly and Triastek joined forces to analysis and develop 3D printed oral medicine for the gastrointestinal tract. The undertaking leveraged Triastek’s proprietary MED know-how to manufacture programmed drug launch profiles, focusing on particular areas of the human digestive system. Dr. Senping Cheng, the Founder, and CEO of Triastek, expressed that the collaboration with Lilly demonstrates the appliance of MED know-how to reinforce drug supply through the oral route. The corporate envisions that the utilization of MED know-how can deal with formulation challenges and result in the event of clinically vital merchandise for its worldwide companions.
Researchers from the MERLN Institute, College of Santiago de Compostela, College Faculty London (UCL), and the UCL spin-out FabRx devised a method to 3D print tablets in simply seven seconds. In contrast to the standard vat photopolymerization course of used for layer-by-layer printing of capsules, the crew employed a volumetric 3D printing method that cured full vats of resin in a single run. This development had the potential to speed up the manufacturing price of personalized remedy, proving to be a essential consider making end-use scientific 3D printing extra possible.
What does the way forward for 3D printing for the following ten years maintain?
What engineering challenges will must be tackled within the additive manufacturing sector within the coming decade?
To remain updated with the newest 3D printing information, don’t overlook to subscribe to the 3D Printing Trade publication or comply with us on Twitter, or like our web page on Fb.
When you’re right here, why not subscribe to our Youtube channel? That includes dialogue, debriefs, video shorts, and webinar replays.
Are you searching for a job within the additive manufacturing business? Go to 3D Printing Jobs for a choice of roles within the business.
Featured picture exhibits Triastek receives FDA IND clearance for its T21 drug. Picture through Triastek.

