In a medical 3D printing breakthrough, the primary US surgical procedures that includes a revolutionary spinal implant comprised of Evonik‘s VESTAKEEP® i4 3DF PEEK filament biomaterial had been carried out earlier this month. Developed by Curiteva, a US-based know-how agency, the implant has been authorized by the US Meals and Drug Administration and is the first-ever 3D printed, absolutely interconnected porous polyether ether ketone (PEEK) implant of its sort for business use.
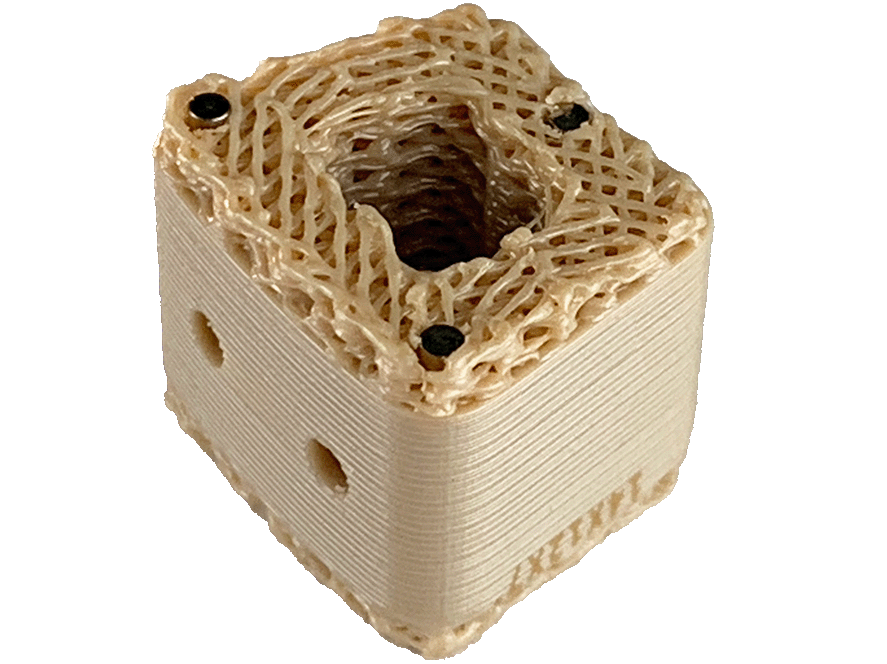
German chemical firm Evonik first introduced the i4 3DF PEEK materials in 2018 and business availability started in 2020.
The groundbreaking surgical procedures came about in mid-April, using the Encourage platform manufactured by Curiteva with Evonik’s VESTAKEEP i4 3DF PEEK high-performance polymer. The proprietary, patented 3D printer answerable for creating the implant was additionally designed, programmed, and constructed by Curiteva, an US firm based in 2017.
Dr. Alex Vaccaro, President of Philadelphia-based Rothman Orthopedic Institute, lauded the event, stating that the lattice PEEK structure enabled by Curiteva’s 3D printing course of represents a major development in backbone, orthopedics, and neurosurgical procedures involving biologic implants.
Equally, Dr. Kevin Foley, Chairman of Semmes-Murphey Neurologic and Backbone Institute and professor of neurosurgery, orthopedic surgical procedure, and biomedical engineering on the College of Tennessee Well being Science Middle, praised the Encourage porous PEEK know-how, highlighting its spectacular interconnected porosity, modulus of elasticity equal to cancellous bone, robust biomechanical properties, radiolucency, and bioactive floor for osseointegration.
PEEK 3D printing filament for surgical implant functions
Evonik’s VESTAKEEP i4 3DF PEEK filament biomaterial, designed particularly for additive manufacturing processes, meets the stringent necessities of ASTM F2026, the usual for PEEK polymers authorized for surgical implant functions. Marc Knebel, Head of Evonik’s Medical Gadgets & Programs market section, emphasised the filament’s distinctive biocompatibility, biostability, and X-ray transparency, making it a really perfect materials for orthopedic and maxillofacial surgical procedure.
As a number one producer in high-performance polymers and components for over 20 years, Evonik’s merchandise have been instrumental in 3D printing functions. The corporate additionally produces a testing-grade PEEK filament and different 3D printing supplies for varied demanding environments, together with resins for photocuring and powders for sintering-based manufacturing processes.

Supplies for medical functions of 3D printing
Elsewhere, Dutch PEEK 3D printing start-up Bond3D and medical implant developer Invibio Biomaterial Options are working collectively on next-generation spinal cages that facilitate higher affected person restoration. These spinal cages not solely retain the therapeutic advantages of their predecessors but additionally possess the required porosity to stimulate new bone formation. The cages are at present present process regulatory steps to achieve FDA approval.
Bond3D specializes within the manufacturing of practical parts from high-performance polymers for vital functions in medical, aerospace, vitality, and automotive markets. Working with Invibio Biomaterial, Bond3D has developed highly-porous spinal cage 3D printing capabilities that allow the creation of fourth-generation implants with enhanced biomechanical and biocompatibility properties to help bone regrowth. The businesses are within the ultimate levels of submitting an FDA utility for one in all these gadgets in partnership with a US-based spinal implant developer.
Along with polymers, current advances in using 3D printed ceramics for medical functions are advancing. Lately, 3D Printing Business spoke to Johannes Homa, CEO of Lithoz, who emphasised the significance of uncompromised materials high quality in 3D printed ceramics. Additionally, within the interview Daniel Bomze, Director of Medical Options at Lithoz, mentioned the corporate’s improvement of bone substitute supplies, corresponding to Lithabone HA 480, which is chemically equivalent to human bones and presents glorious biocompatibility and osseoconductivity. Because the 3D printed ceramic implant dissolves, it’s changed by the affected person’s pure bone materials. Lithoz’s medical ceramic functions embody patient-specific cranial implants and bone substitute for vital dimension defects in lengthy bones.
Featured picture reveals 3D printed PEEK spinal implant. Picture through Curiteva.
To remain updated with the most recent 3D printing information, don’t overlook to subscribe to the 3D Printing Business publication or observe us on Twitter or liking our web page on Fb.
Whilst you’re right here, why not subscribe to our Youtube channel? that includes dialogue, debriefs, video shorts and webinar replays.
Are you searching for a job within the additive manufacturing business? Go to 3D Printing Jobs for a collection of roles within the business.
